生殖毒性
根据动物研究,西尼莫德可能会伤害胎儿。育龄妇女应在接受西尼莫德治疗期间和停止西尼莫德治疗后 10 天内采取有效避孕措施以避免怀孕1,2

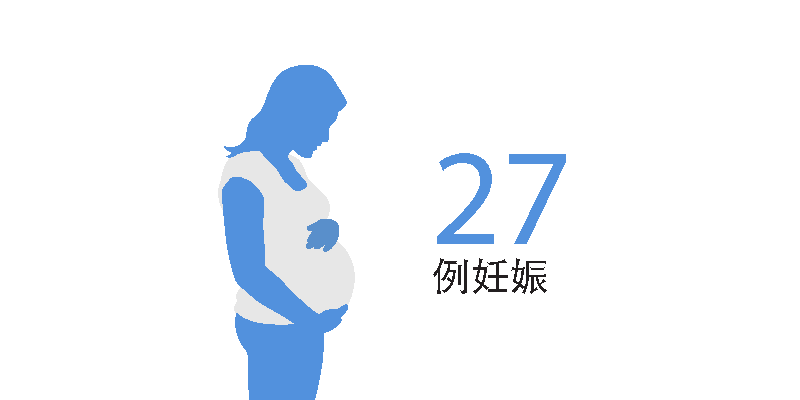
截至 2022 年 3 月 25 日4,上市后环境中没有接受西尼莫德的患者报告妊娠
- 有 26 名孕妇在妊娠期间暴露于西尼莫德;5 名妇女在妊娠前暴露于西尼莫德3
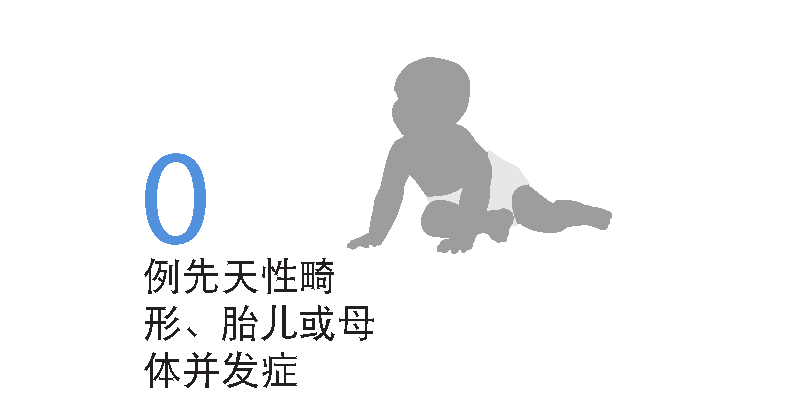
暴露于西尼莫德的孕妇未报告先天性畸形(包括重度和轻度)和与妊娠和分娩相关的胎儿或母体并发症病例3

It looks like you are using an older version of Internet Explorer which is not supported. We advise that you update your browser to the latest version of Microsoft Edge, or consider using other browsers such as Chrome, Firefox or Safari.
根据动物研究,西尼莫德可能会伤害胎儿。育龄妇女应在接受西尼莫德治疗期间和停止西尼莫德治疗后 10 天内采取有效避孕措施以避免怀孕1,2


截至 2022 年 3 月 25 日4,上市后环境中没有接受西尼莫德的患者报告妊娠

暴露于西尼莫德的孕妇未报告先天性畸形(包括重度和轻度)和与妊娠和分娩相关的胎儿或母体并发症病例3

This website is for non-promotional purposes and is intended for providing
safety information for healthcare professionals (HCPs) Only
Please confirm that you are an HCP
For HCPs: Information on this website is not country specific, and may contain information that is outside the approved indications in the country in which you are located. Please contact your local Novartis representative for the latest information specific to your country.
For non-HCPs / patients: This safety website is available for HCPs only
妊娠结局强化监测 (PRIM) 计划基于 Novartis 自发报告系统的增强药物警戒。PRIM 是一项不良事件结局强化监测计划,旨在收集妊娠即将开始前或妊娠期间暴露于西尼莫德患者的妊娠信息(目标随访清单),以及妊娠 12 个月后的婴儿结局。