BOLD 延展研究
西尼莫德对剂量滴定的影响
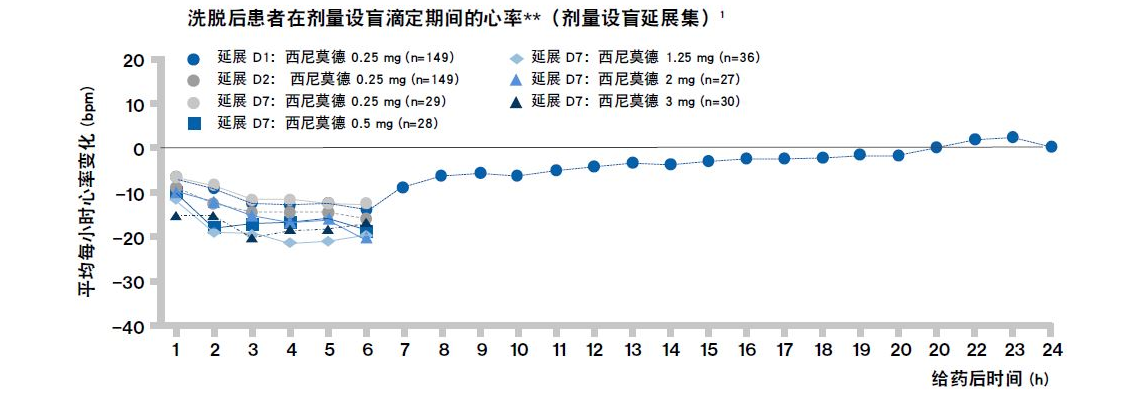
剂量滴定可有效降低 BOLD 研究中较高剂量下观察到的西尼莫德的负性变时性效应
**洗脱患者是指在 BOLD 研究中最后一次服用研究药物日期与延展研究药物首次给药日期之间研究药物中断超过 7 天的患者,以及在 BOLD 研究中随机分配至安慰剂组的所有患者
BOLD 延展是 II 期 BOLD 研究的给药设盲延展阶段,持续至多 24 个月,随后是开放性西尼莫德治疗,确定安全性和疗效,并评估西尼莫德剂量滴定对治疗开始时心率变化的缓解作用

It looks like you are using an older version of Internet Explorer which is not supported. We advise that you update your browser to the latest version of Microsoft Edge, or consider using other browsers such as Chrome, Firefox or Safari.
西尼莫德对剂量滴定的影响

剂量滴定可有效降低 BOLD 研究中较高剂量下观察到的西尼莫德的负性变时性效应

This website is for non-promotional purposes and is intended for providing
safety information for healthcare professionals (HCPs) Only
Please confirm that you are an HCP
For HCPs: Information on this website is not country specific, and may contain information that is outside the approved indications in the country in which you are located. Please contact your local Novartis representative for the latest information specific to your country.
For non-HCPs / patients: This safety website is available for HCPs only
妊娠结局强化监测 (PRIM) 计划基于 Novartis 自发报告系统的增强药物警戒。PRIM 是一项不良事件结局强化监测计划,旨在收集妊娠即将开始前或妊娠期间暴露于西尼莫德患者的妊娠信息(目标随访清单),以及妊娠 12 个月后的婴儿结局。