Real World Evidence
UCSF Longitudinal study: interim analysis
Sabatino J et al. Longitudinal Analysis of Adaptive Immunity Following Additional SARS-CoV-2 Vaccination in MS Patients on Anti-CD20 Therapies and Sphingosine-1-phosphate Receptor Modulators
Figure: Antibody responses to SARS-CoV-2 vaccination for the different groups at each time point
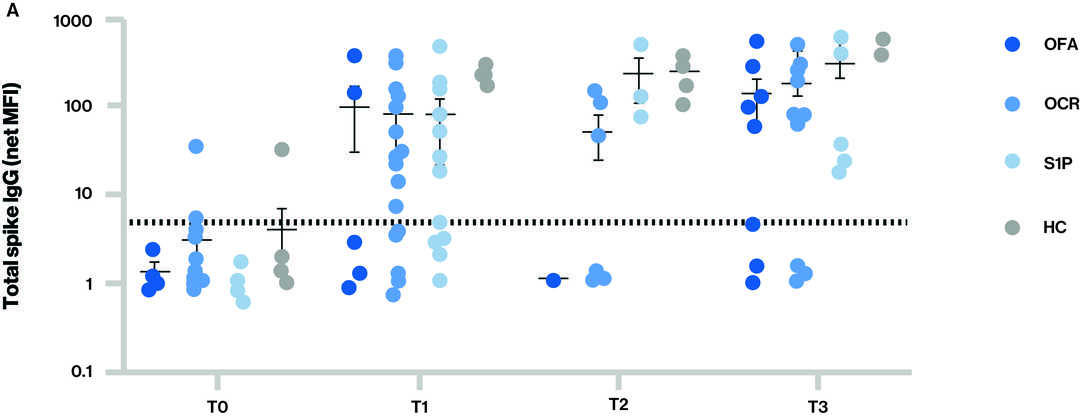
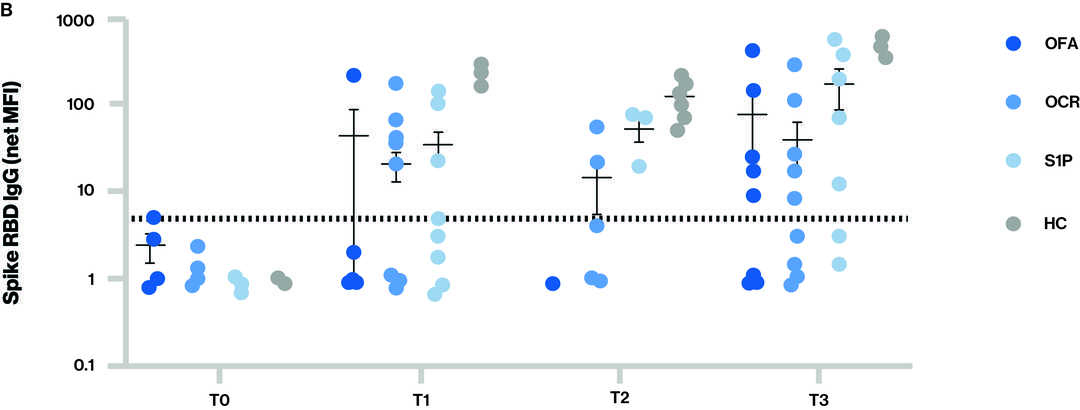
The dotted line in figure A and B indicates the cut-off for seropositivity; Time points (T) at blood sample collection: T0 - Baseline, T1 – Sample collected after receiving full two dose vaccination series, T2 – Sample collected prior to 3rd vaccine (booster) and T3 – Sample collected after 3rd vaccine (booster); S1P-(fingolimod and siponimod)
- IgG levels to total spike (A) and spike (receptor binding domain) RBD (B) for the different groups at each time point.
- Seropositivity at T3 (at least 14 days after the third dose of mRNA vaccine):
- For total spike IgG, which includes activity against any epitope on spike protein that may not correlate to neutralizing antibodies, 62.5% (5/8) ofatumumab, 75.5% (9/12) ocrelizumab,100% (7/7) S1P, and 100% (7/7) healthy volunteers
- For spike RBD IgG, which is more specific and a better correlate of neutralizing antibodies, 62.5% (5/8) ofatumumab, 50.0% (6/12) ocrelizumab, 71.4% (5/7) S1P, and 100% (7/7) healthy volunteers
Figure T-cell response – Proportion of CD4+ T cells (A) and CD8+ T cells (B) that are spike-reactive are shown for the different treatment groups at each time point
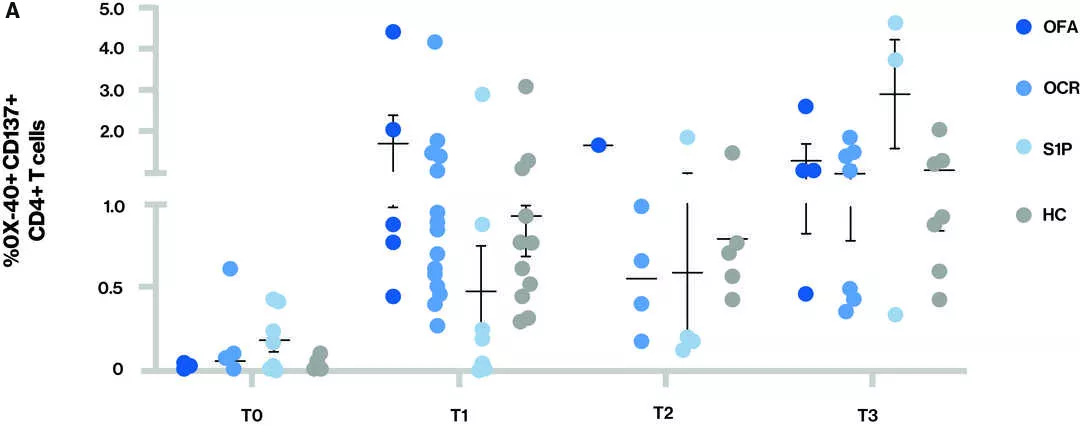
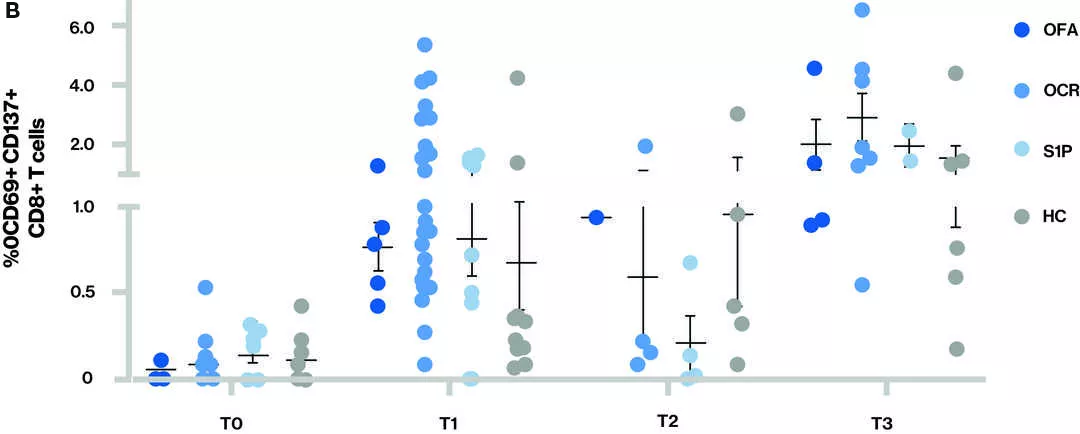
Time points (T) at blood sample collection: T0 - Baseline, T1 – Sample collected after receiving full two dose vaccination series, T2 – Sample collected prior to 3rd vaccine (booster) and T3 – Sample collected after 3rd vaccine (booster); S1P- (fingolimod and siponimod)
- At T3, all participants appeared to have both a CD4 and CD8 T-cell response
In this interim analysis of adult MS patients on S1Ps (fingolimod and siponimod) and anti-CD20s(ocrelizumab and ofatumumab), while immune response was overall attenuated, a majority of patients in all DMT groups (> 70% S1P and > 50% anti-CD20) mounted an antibody response to 3rd vaccination (booster), All adult MS patients on S1Ps and anti-CD20s mounted a T-cell response to 3rd (booster) vaccine in all DMT groups