EXCHANGE
Interim analysis results1
Bar-Or A, et al. , Safety and Tolerability of Conversion to Siponimod With and Without Titration in Patients with Advancing Forms of RMS: Interim Results of the Phase 3b EXCHANGE Study. Poster presentation at AAN 2022, P7.007
- EXCHANGE evaluated safety and tolerability of immediate conversion to dose-titrated siponimod from other DMTs in patients with advancing RMS
- Prospective, multicenter, open-label, single-arm trial
- 163 patients included in interim analysis from 42 centers in the USA
- Patients with ≥1 AE (31.3%) possibly related to siponimod treatment
- There was no decrease in heart rate at 6 hours post first dose from baseline in the overall or any of the prior DMT groups
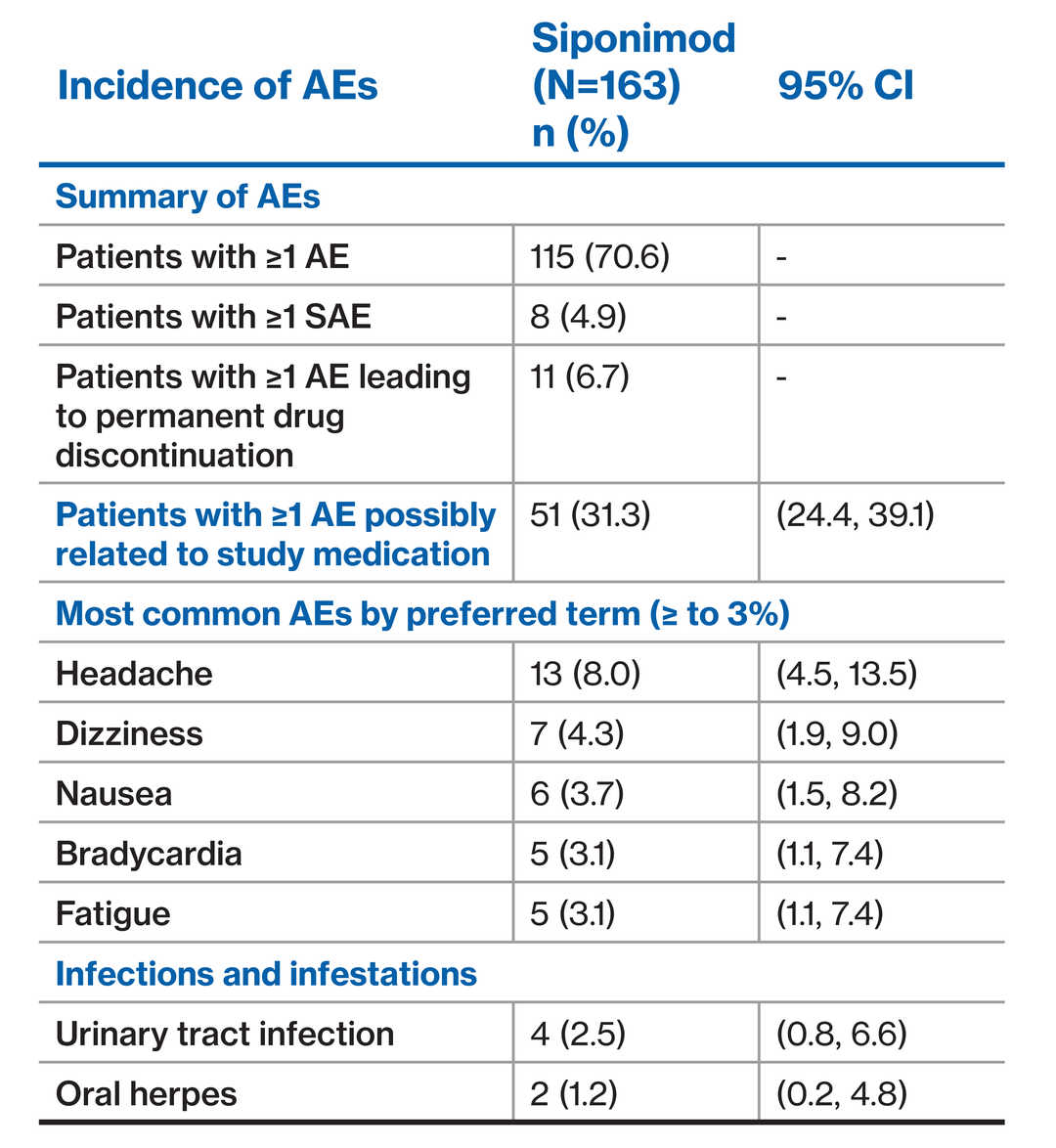
Immediate conversion over 6 days from other DMTs to siponimod was generally well tolerated, with no unexpected findings
Bar-Or A, et al., Safety and Tolerability of Conversion to Siponimod in Patients with Advancing Relapsing Multiple Sclerosis: A Subgroup Analysis by Race and Ethnicity of EXCHANGE Interim Data, Poster presentation at ACTRIMS 2022, P109
- EXCHANGE study enrolled a diverse patient population and presents opportunity to assess MS treatment patterns and safety/tolerability in conversion to siponimod
- Prospective, multicenter, open-label, single-arm trial
- Of 163 patients in the overall EXCHANGE interim population 126 (77.3%) identified as White, non-Hispanic/Latino – 23 (14.1%) identified as Black/African American – 36 (22.1%) identified as Hispanic/Latino
- Mean heart rate at baseline and 6-hour post first dose in both patient subgroups were comparable to the findings observed in the overall EXCHANGE interim population
- The most common AE related to siponimod treatment by preferred term was headache in the overall population (n=13; 8.0%), 11 of whom were Hispanic/Latino
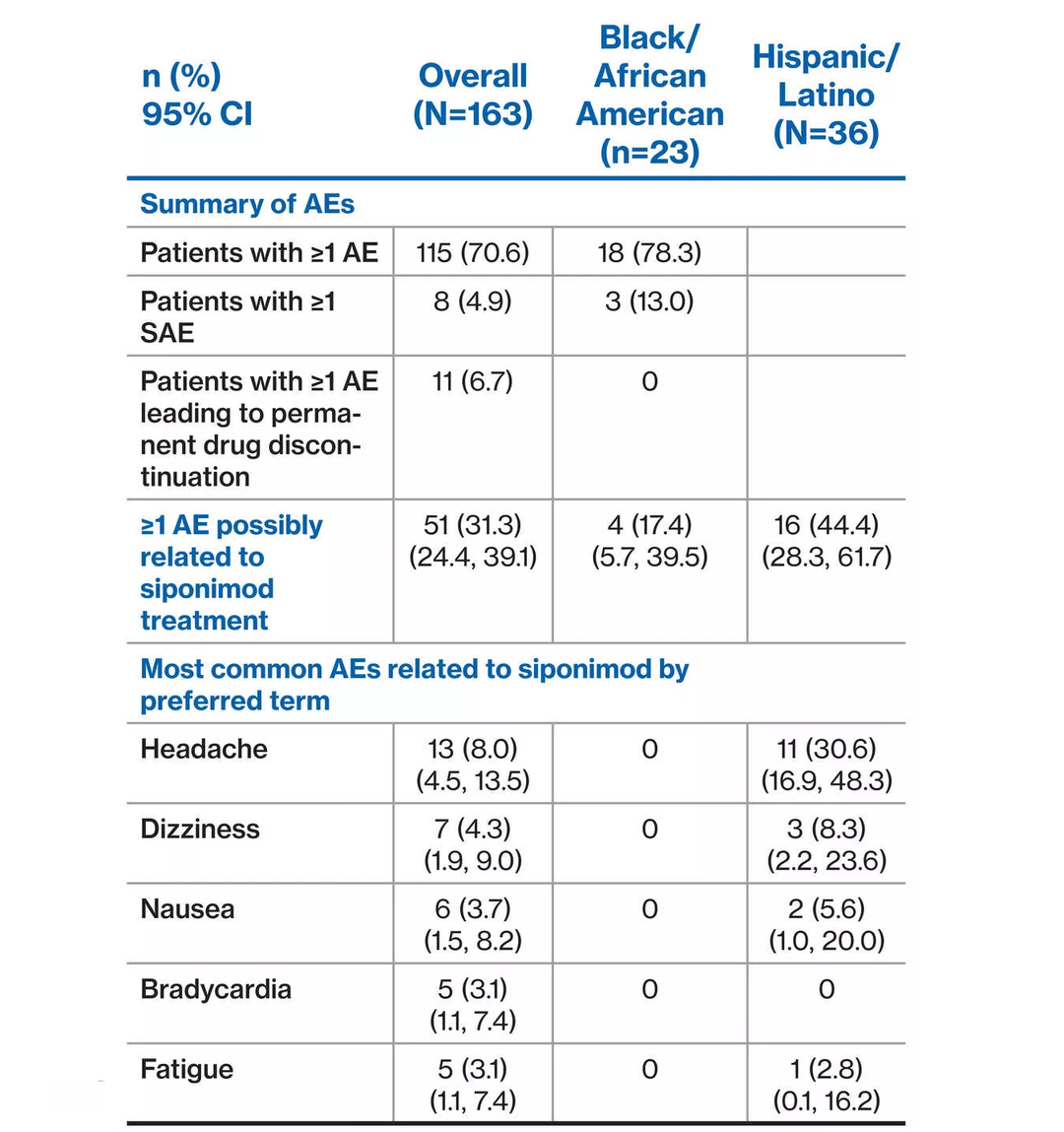
Findings of this subgroup analysis by race/ethnicity provide some insights into treatment patterns and safety/tolerability in minority MS patient populations. Siponimod safety/tolerability profile remained consistent with no new or unexpected safety findings identified through this analysis
Bar-Or A, et al., Evaluating Humoral Immune Response to mRNA COVID-19 Vaccines in Siponimod-treated Patients with Advancing Forms of Relapsing Multiple Sclerosis: A COVID-19 Vaccine Sub-study of Phase 3b EXCHANGE Trial, Poster presented at ACTRIMS 2022, P133
- EXCHANGE is a 6-month, open-label, single-arm Phase 3b trial of safety and tolerability of immediate conversion to dose-titrated SIPO from other DMTs in patients with advancing RMS
- To report results of a sub-study assessing humoral immune response to mRNA COVID-19 vaccines (Pfizer/Moderna) in a subset of patients enrolled in EXCHANGE
- Overall, 70% (7/10) achieved a positive humoral immune response to COVID-19 vaccine at the post-vaccination assessment
- 57.1% (4/7) and 100% (3/3) achieved a positive response after two and three vaccine doses, respectively
Immune response to COVID-19 vaccine
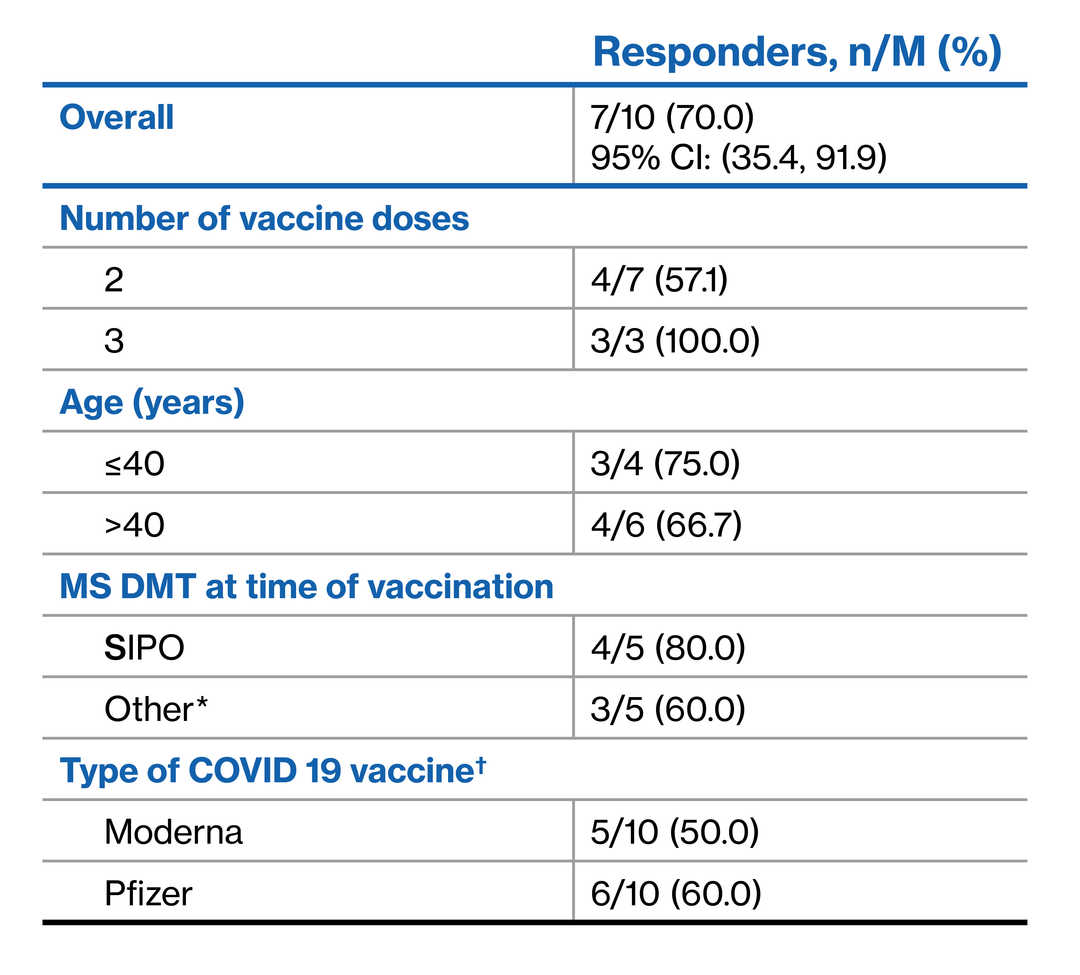
Albeit limited by small sample size, this preliminary sub-study adds to our understanding of humoral immune responses to mRNA COVID-19 vaccination in patients with advancing forms of RMS who switched to SIPO treatment
Abbreviations
AEs, adverse events; CI, confidence interval; DMTs, disease-modifying therapies; RMS, relapsing multiple sclerosis; SAEs, serious adverse events**^