Reproductive toxicity
Based on animal studies, siponimod may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during and for 10 days after stopping siponimod treatment1,2
| If pregnancy test shows positive, please contact the registry |


As of March 25, 2024, 47 pregnancies (34 prospectivea and 13 retrospectiveb) were reported from Novartis safety database with siponimod
aProspective cases are defined as cases for which, at the time of initial reporting (i.e., first receipt by Novartis), the pregnancy outcome has not yet occurred or there is no report of an abnormal prenatal testing result (including cases where prenatal testing has not yet been performed, or cases where prenatal testing has been performed but results were either normal or not specified
bRetrospective cases are defined as cases for which at the time of initial reporting (i.e., first receipt by Novartis), the pregnancy outcome has already occurred, or prenatal testing results were abnormal (regardless of whether the pregnancy outcome has occurred)
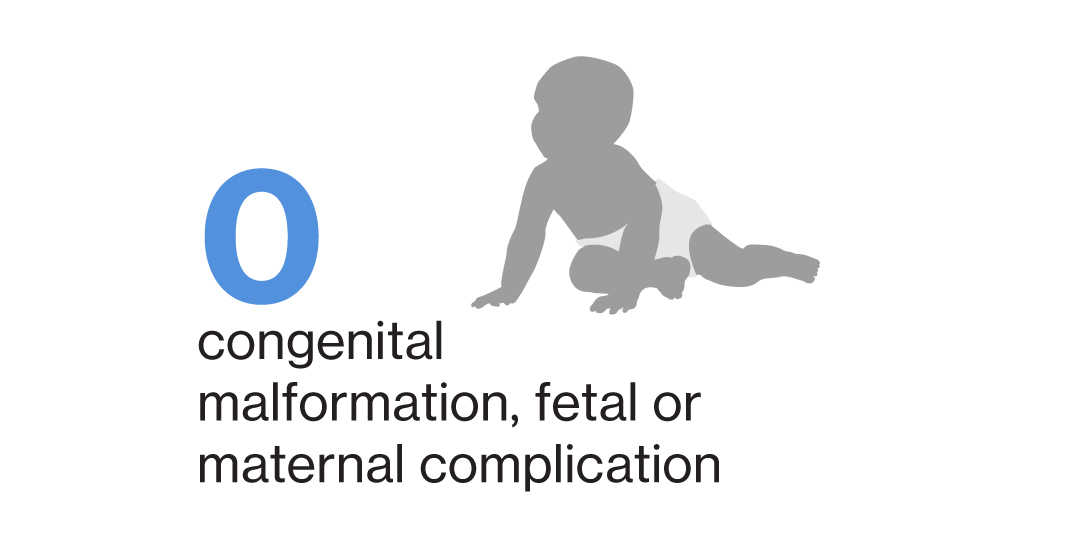
No events of congenital malformation (including major and minor) and fetal or maternal complication related to pregnancy and delivery have been reported in pregnant women exposed to siponimod3
