AMA-VACC
Interim analysis results1
Tjalf Ziemssen, et al. Assessing the immune response to SARS-CoV-2 mRNA vaccines in siponimod-treated patients: a nonrandomized controlled clinical trial (AMA-VACC); Therapeutic Advances in Neurological Disorders, November 8, 2022; https://doi.org/10.1177/1756286422113530
Data presented are for patients with evaluable antibody and T-cell assessments
- AMA-VACC clinical trial was designed to characterize immune responses to SARS-CoV-2 mRNA vaccines in siponimod-treated SPMS patients
- The study assessed the immune response in siponimod treated SPMS patients after initial and booster SARS-CoV-2 mRNA vaccination
- Prospective, multicenter, open-label, three-cohort trial
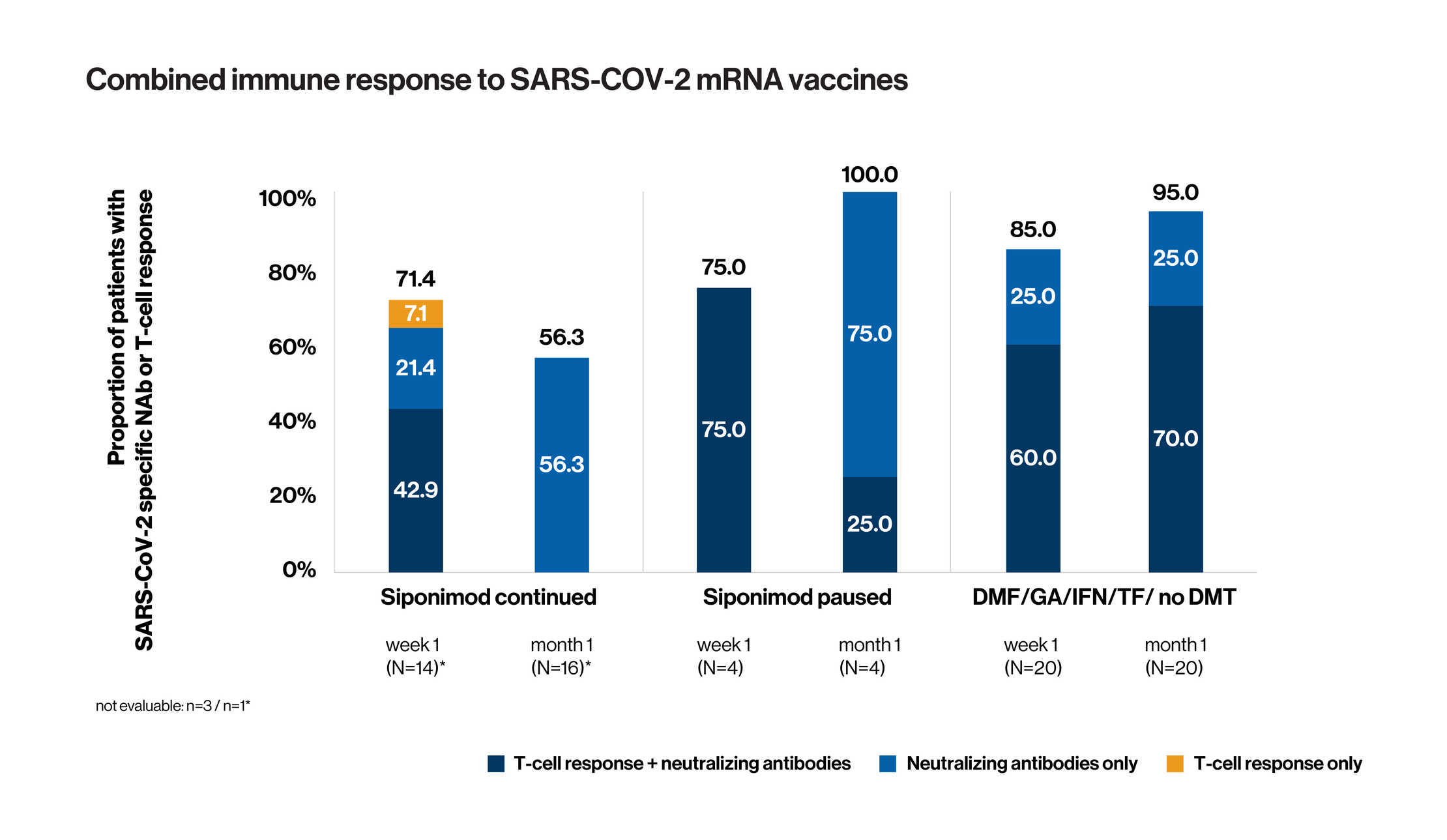
- 41 patients included in interim analysis (17 in cohort 1: ongoing siponimod treatment during vaccination; 4 in cohort 2: siponimod interrupted for vaccination; and 20 patients in cohort 3: first line DMT/no current treatment)
- NAb at 1 week was reached by 52.9% in continuous siponimod treatment cohort, 75.0% in interrupted siponimod cohort and 90% of cohort 3
- Seroconversion at month 1 was observed in 56.3%, 100% and 95% in cohort 1, 2 and 3, respectively
- One week after vaccination, 50%, 75% and 60% of the patients in cohort 1, 2 and 3, respectively, mounted SARS-CoV-2 specific T-cell response. After 1 month, T-cell reactivity was observed in 0.0%, 25% and 70% of cohort 1, 2 and 3, respectively.
- Analysis of combined immune response (development of SARS-CoV-2-specific NAbs or T-cell reactivity or both) showed that 1 week after the second dose of vaccine, 71.4% of cohort 1, 75.0% of cohort 2, and 85.0% of cohort 3 were positive for either humoral or cellular response or both. One month after the second dose of vaccine, 56.3% of cohort 1, 100.0% of cohort 2, and 95.0% of cohort 3 were positive for either humoral or cellular response or both
- No COVID-19 infection was reported, and no deaths were reported until the cut-off date
The interim analysis shows that majority of the siponimod treated patients mounts humoral and cellular response under continuous siponimod cohort.