EXPAND
Safety
EXPAND population: Overall safety profile1-3
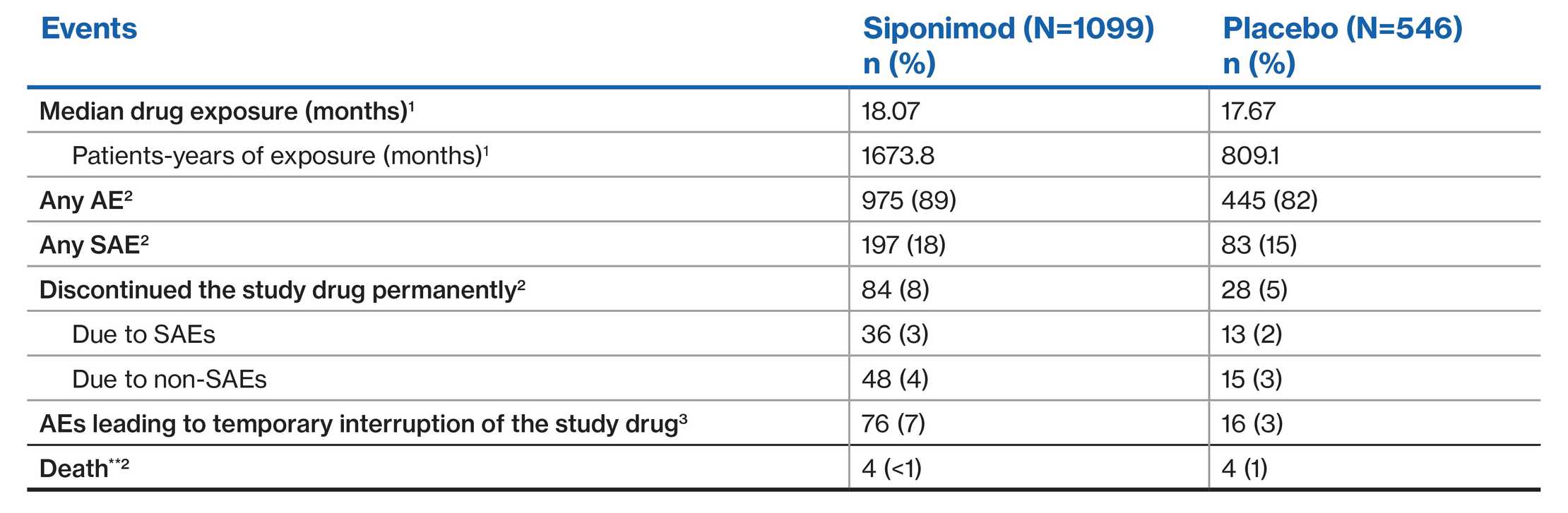
**Deaths in the siponimod group were due to metastatic gastrointestinal melanoma within 4 months of commencing siponimod; septic shock in a patient with terminal colon cancer; urosepsis more than 10 weeks after discontinuation of siponimod and after two doses of rituximab; and suicide. One additional patient with metastatic lung carcinoma withdrew consent from the study after having been on siponimod for 11 months; this patient died (unspecified reason) about 5 months after discontinuing the study medication. Deaths in the placebo group were due to haemorrhagic stroke, lung cancer, gastric cancer, and for an unknown reason

EXPAND population: Most common AEs (≥5% of patients in either group)2
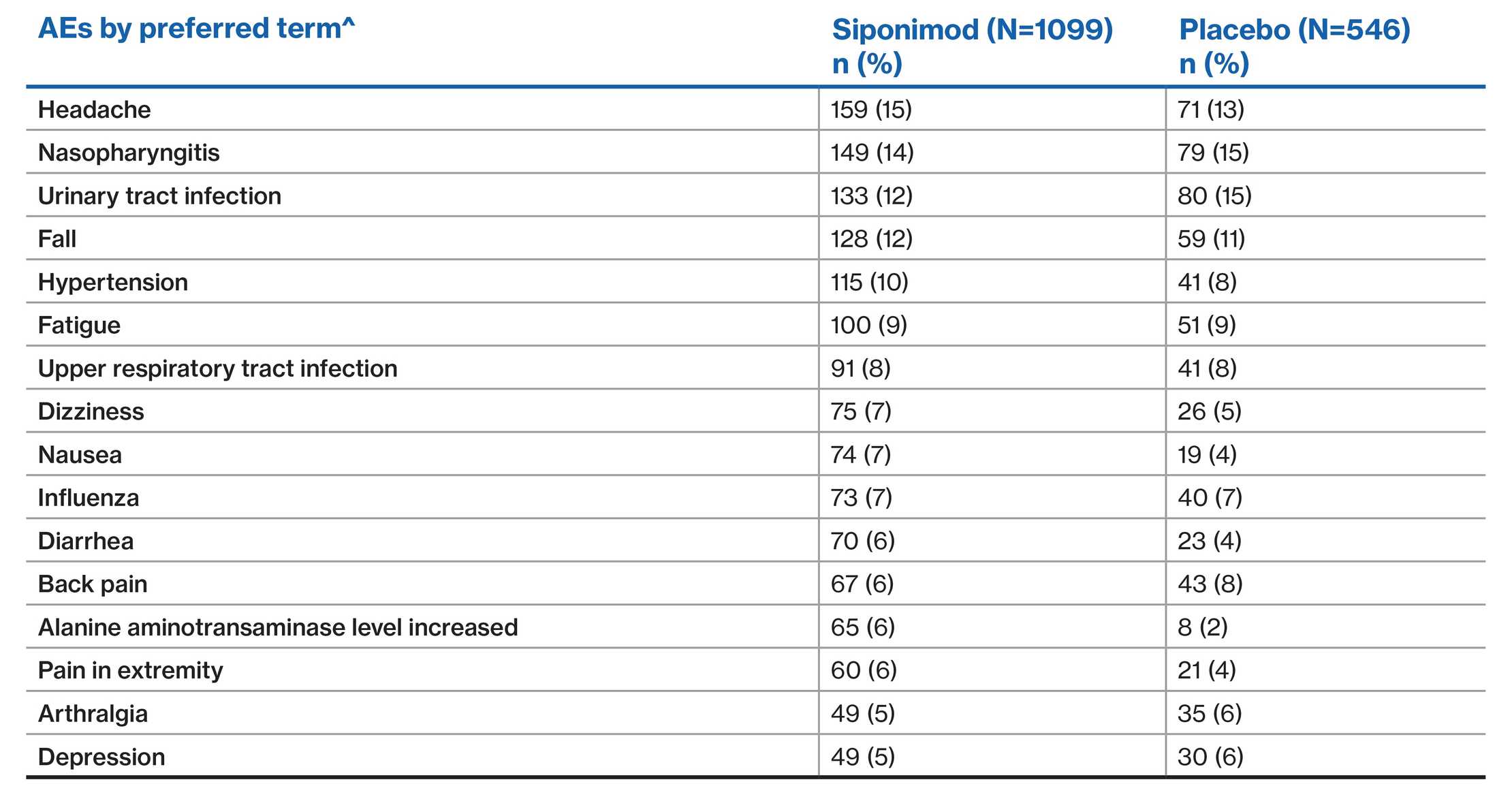
^AEs were coded according to the MedDRA, version 19·0
Data presented from the EXPAND placebo-controlled Phase 3 study

EXPAND population: SAEs (≥0.5% of patients in either group)2
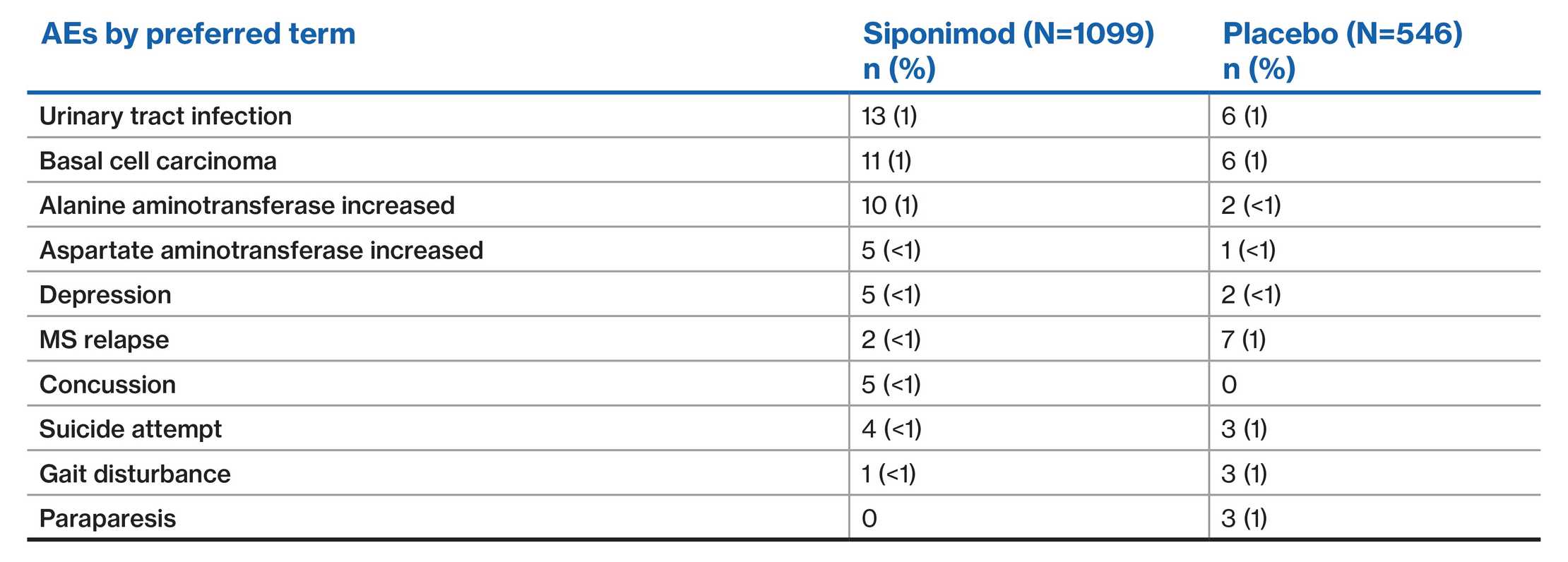
Data are presented from the EXPAND placebo-controlled Phase 3 study

AEs of special interest
- AEs of special interest include well known pharmacodynamic effects of the S1P modulators, risks associated with immunosuppression, and some events that occurred with S1Ps but for which the mechanism remains unclear
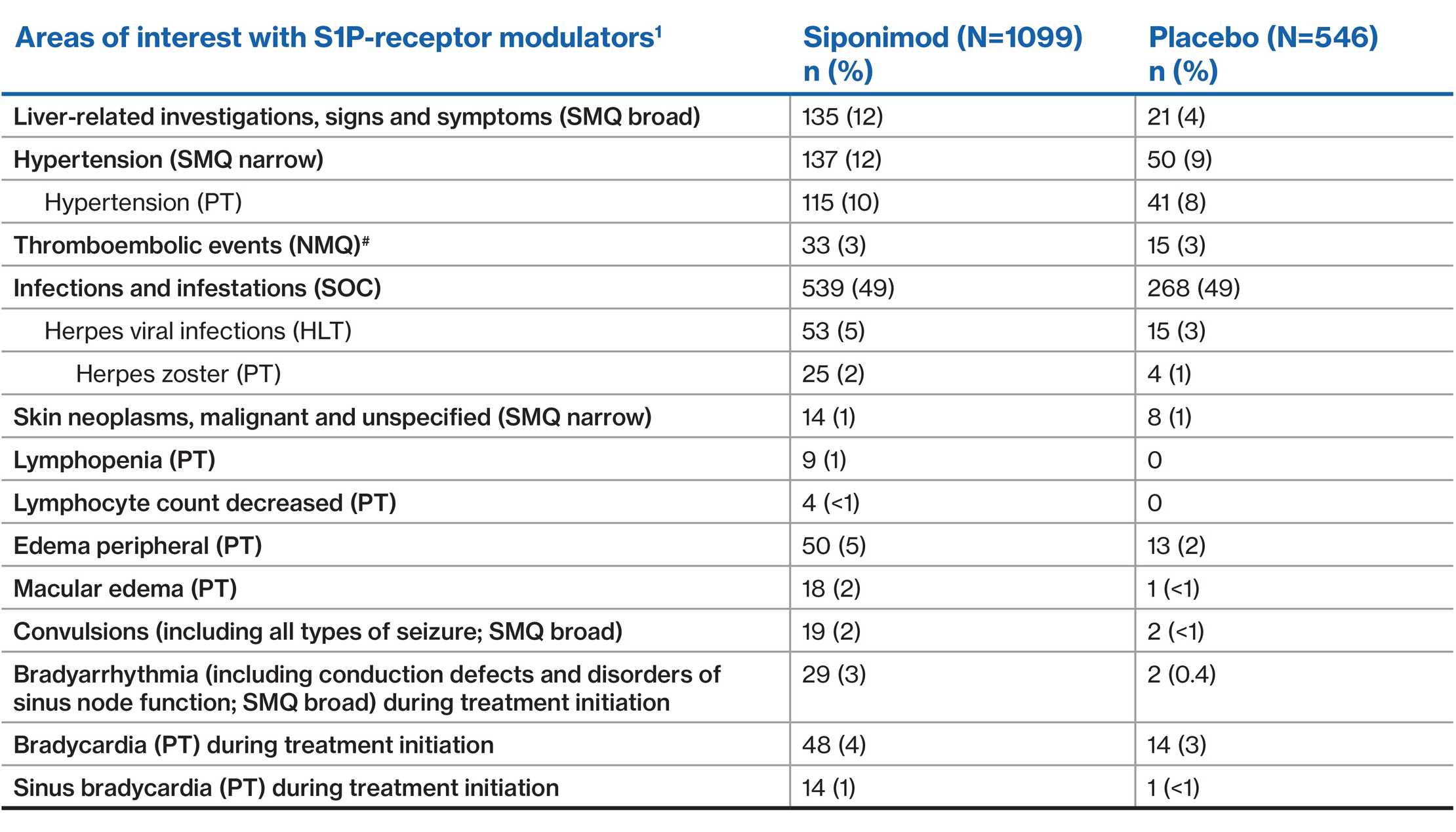
#Include 'central nervous system hemorrhages and cerebrovascular conditions (SMQ broad)', 'embolic and thrombotic events, arterial (SMQ)', and 'ischemic heart disease (SMQ broad)'
