Infections
The immune system effects of siponimod may increase the risk of infections. A core pharmacodynamic effect of siponimod is a dose-dependent reduction in peripheral lymphocytes due to the reversible sequestration of lymphocytes in lymphoid tissues
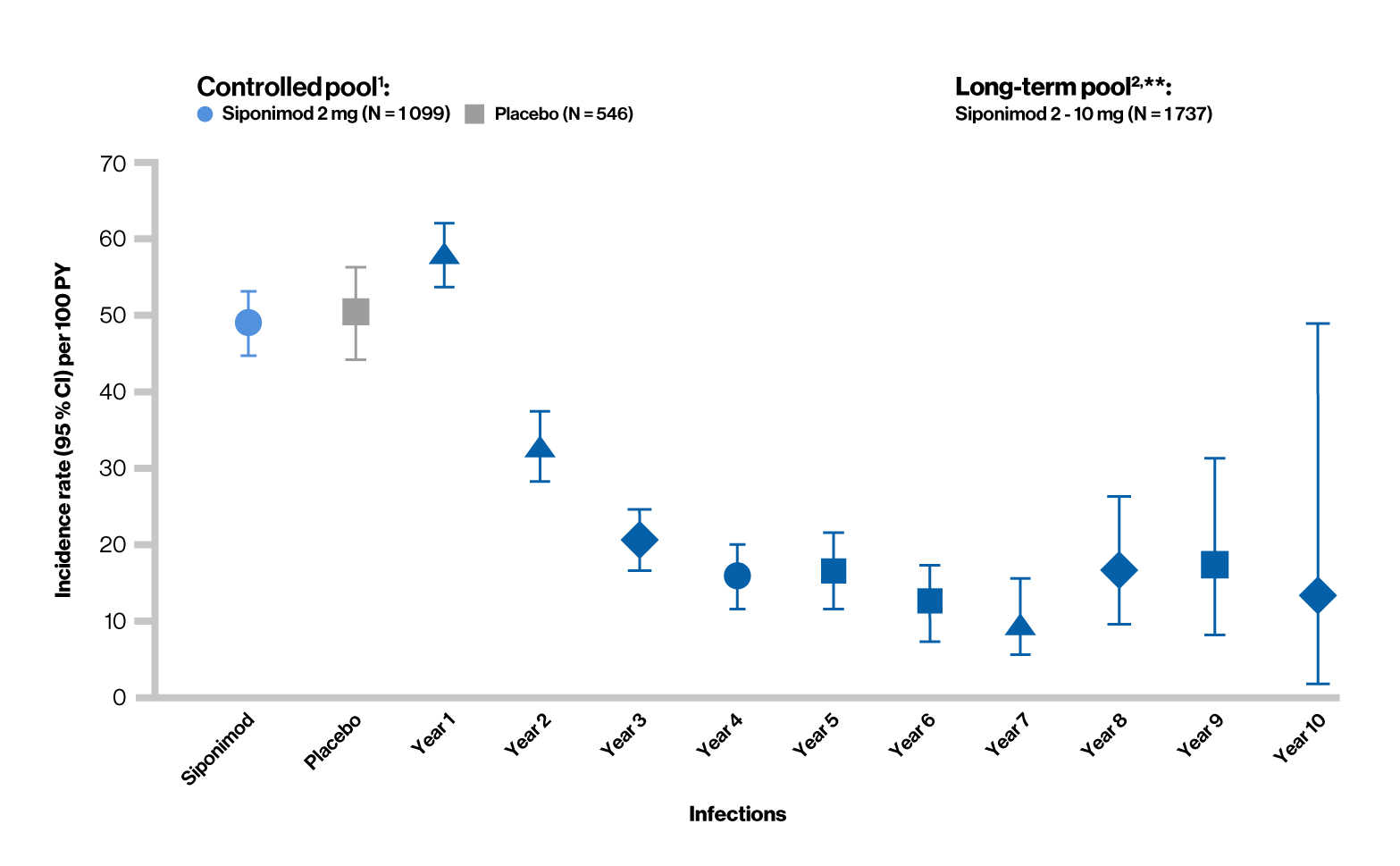
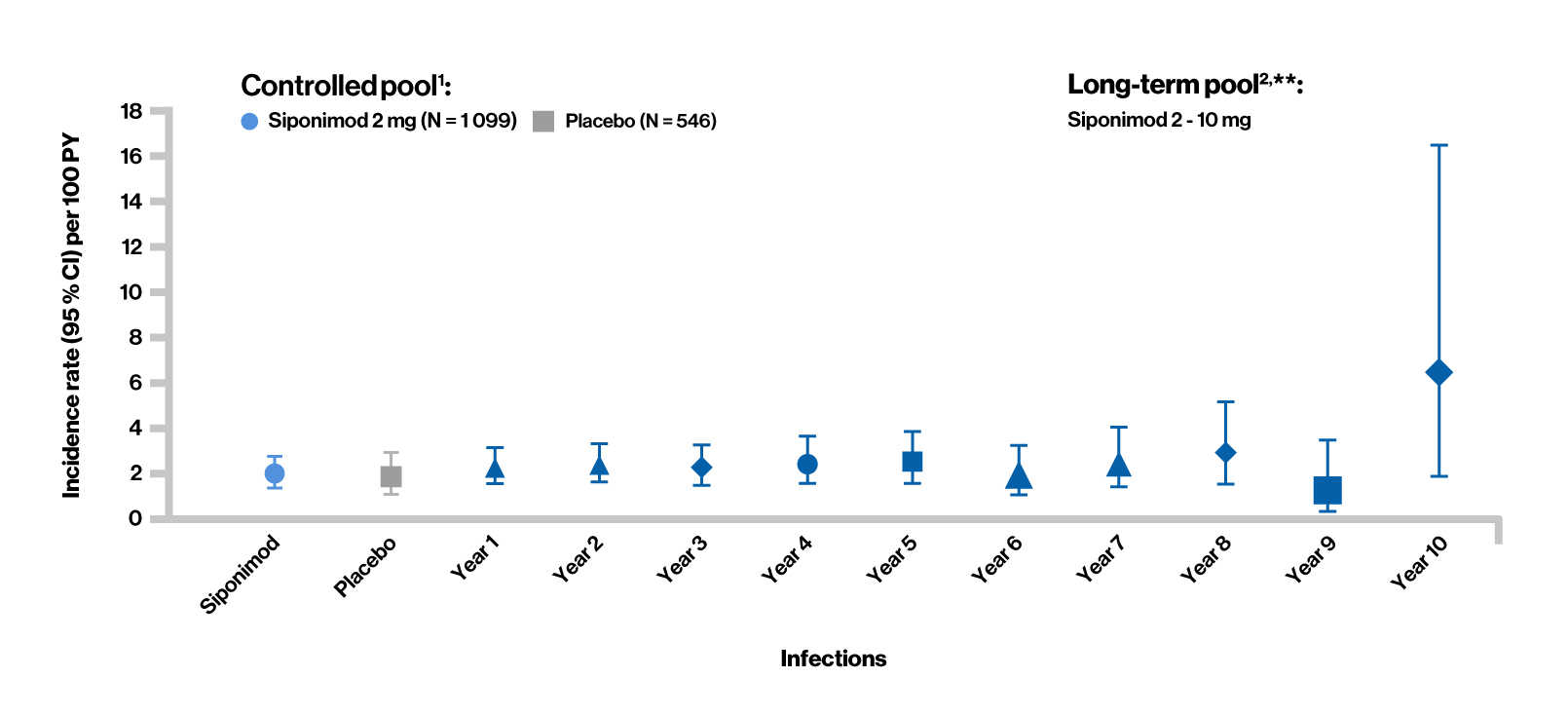
Overall infection rates in siponimod clinical trials
Controlled pool1 (median exposure: 17 months)
- In the controlled pool, the overall rate of infections was similar between the siponimod and placebo groups
- The risk of serious infections with siponimod was similar to that with placebo
- Nasopharyngitis, UTI, URTI, and influenza were the most commonly reported infections
- No particular pattern of infections was identified, and the observed events were consistent with events reported with other S1P receptor modulators
Long-term pool2 (median exposure: 59 months)
- As of March 31, 2023, no increase in the overall incidence of infections (treatment-emergent AE and treatment-emergent serious AE) was observed in siponimod-treated patients, which were consistent with that in the controlled pool. As observed in the controlled period, there was no change in the type or pattern of reported infections over time
- UTI was the most common reported serious AE in the siponimod group
Treatment-emergent adverse events (Infections)
Treatment-emergent serious adverse events (Infections)
**Long-term estimate obtained using cumulative incidence function.
Controlled pool1 included the double-blind, placebo-controlled core part of the BOLD and EXPAND studies. Long-term pool2 included target siponimod treatment period in the core (controlled and open-label) and/or extension phases of the BOLD and EXPAND studies.

Postmarketing experience3
- As of March 25, 2024, siponimod treatment outside of clinical trials corresponds to an exposure of 50,657 patient-years
- The most commonly reported serious infections were UTI, and nasopharyngitis
Infection rates in MS population
- Patients with MS are at an increased risk of infections compared with a matched population without MS4,5,^
- The incidence rate of any infection was higher among patients with MS than those without MS (4805 vs 2731 per 10,000 person-years; IR ratio [95% CI], 1.76 [1.72‒1.80])4
^Study methodology4
The study compares rates of infection in patients with MS after MS diagnosis with a matched population of patients without MS.
The MS cohort included patients who had MS diagnosed and were treated between January 2004 and August 2017.Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. Patients were followed until loss of eligibility, death, or end of data collection. In all, the study included 8695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. The median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with patients without MS (43.9% vs. 36.3%).
