EXPAND
Study design
EXPAND Phase 3 study design: Event-driven design allowed for adequate observation period for the assessment of efficacy and safety while reducing exposure to placebo1
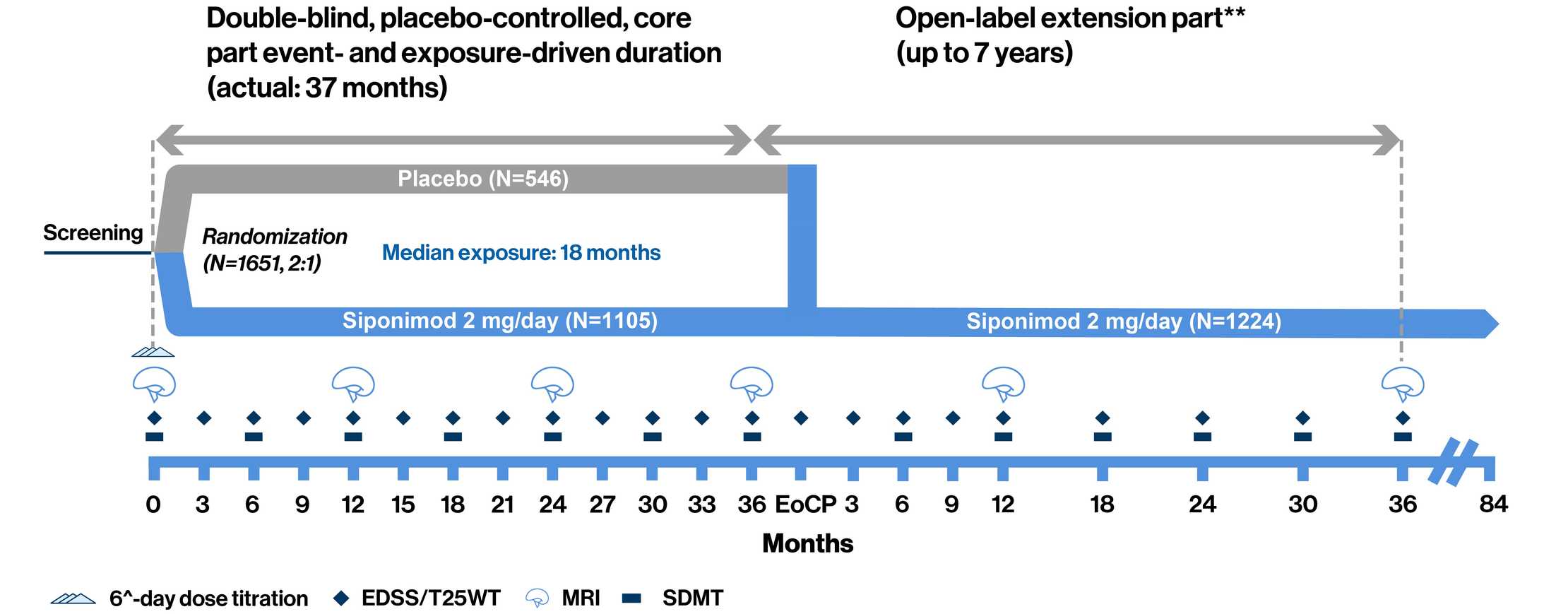
**Open-label part starts when a patient has an 'event'. ^Treatment was initiated with a 6-day titration period (0.25 mg–2 mg) with the first maintenance dose of 2 mg reached on Day 6.
The Phase 3 EXPAND study was a randomized, parallel-group, double-blind, placebo-controlled, event-driven, and exposure-driven core part (up to 3 years) followed by an open-label extension part (up to 7 years), which collected long-term data to evaluate the efficacy and safety of siponimod, for up to 10 years. Patients (aged 18–60 years) with SPMS and EDSS score of 3.0–6.5 were randomly assigned (2:1) to receive once-daily oral siponimod 2 mg or placebo for up to 3 years or until the occurrence of a prespecified number of CDP events. The primary endpoint was time to 3-month CDP. CDP was defined as a 1-point increase in EDSS if the baseline score was 3.0–5.0, or a 0.5-point increase if the baseline score was 5.5–6.5, confirmed at a scheduled visit at least 3 months later. The two key secondary endpoints were time to 3-month CDP of at least 20% from baseline in the T25FW test and change from baseline in T2 lesion volume1
- 374 events of 3mCDP and ≥1 year of exposure for >95% randomized patients were required
- Patients with 6mCDP had an option to switch to open-label siponimod or other DMTs while remaining in the core part of the study
- At the EoCP, patients had the option to enter the still ongoing open-label extension part of the trial and receive siponimod for up to additional 7 years

EXPAND population: Patient demographics and baseline disease characteristics1
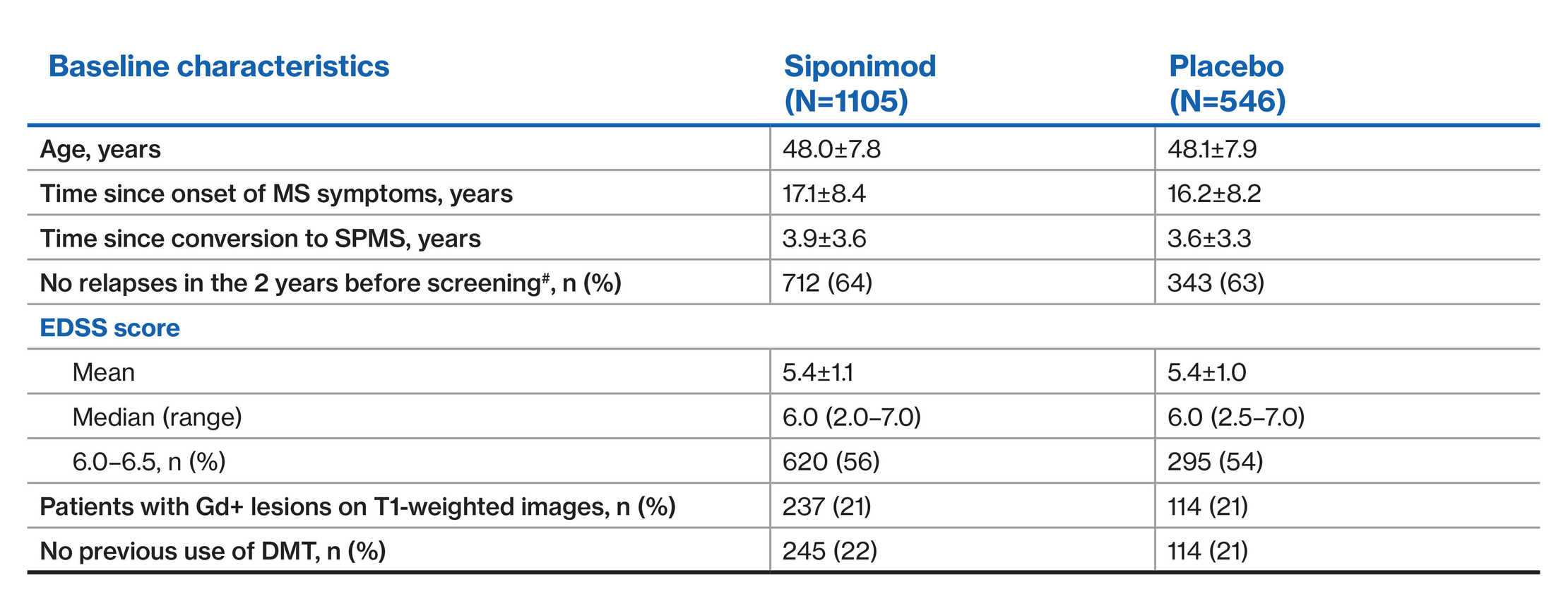
#Data are presented as mean±SD, unless otherwise specified
^Information on the number of relapses in the past 2 years was not available for three patients in the siponimod group and one patient in the placebo group

EXPAND SPMS population and Phase 3 RMS trials: Patient demographics and baseline
disease characteristics1‒7
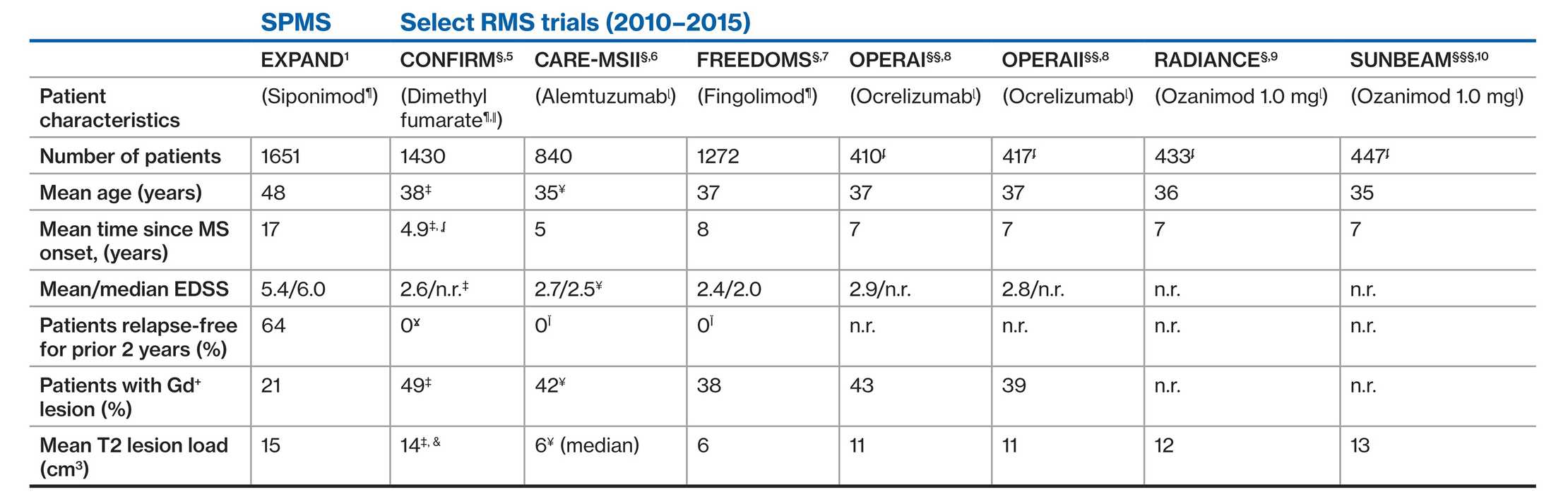
As there are no head-to-head trials of siponimod versus these agents, no comparisons could be made due to differences in study design
§At 2 years; §§At 96 weeks; §§§At 12 months; ¶placebo comparator; ǁglatiramer acetate comparator; ɭIFN β-1a comparator; ¥alemtuzumab 12 mg arm only; ‡Twice daily DMF arm only; ʆtime since diagnosis; ĬEDSS score higher than 5.0; Ɣbased on inclusion criteria; &MRI cohort; ʄInclude data of patient who received ocrelizumab or ozanimod
