BOLD
Effect of siponimod on heart rate
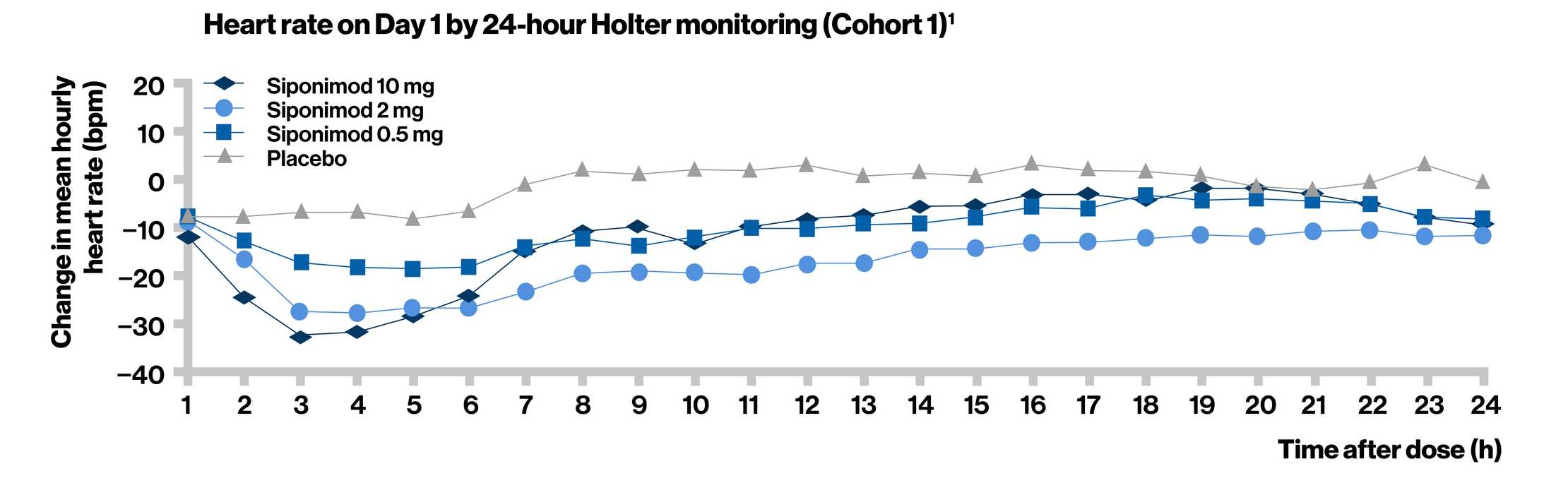
- In Cohort 1, a dose-dependent decrease in heart rate was observed upon treatment initiation
- The effect was most pronounced at approximately 3 to 6 hours after the first dose
- The heart rate returned to pre-dose levels within 24 hours
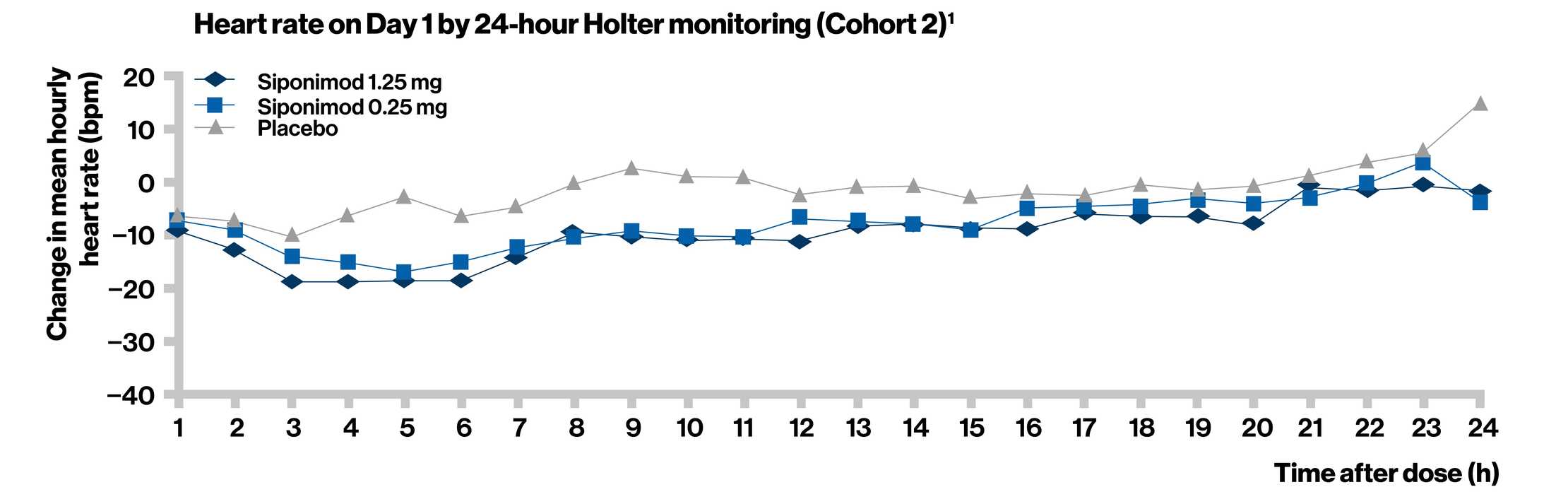
- Treatment initiation with dose titration (siponimod 0.25 mg) mitigated the negative chronotropic and dromotropic effects of siponimod, which are thought to be associated with the modulation of S1P1 on human atrial myocytes
BOLD was a double-blind, adaptive, dose-ranging phase 2 study in adults (aged 18–55 years) with RRMS where two patient cohorts were sequentially tested, separated by an interim analysis at 3 months. The study determined the dose-response relation of siponimod in terms of its effects on brain MRI lesion activity and characterized safety and tolerability in patients with RRMS
Two patient cohorts were sequentially tested, separated by an interim analysis. Patients in cohort 1 were randomly allocated (1:1:1:1) to receive siponimod 10 mg, 2 mg, or 0.5 mg, or placebo once-daily for 6 months. Two additional doses for patients in cohort 2 were selected on the basis of results of an interim analysis of cohort 1 at 3 months. Patients in cohort 2 were randomly allocated (4:4:1) to receive siponimod 1.25 mg, siponimod 0.25 mg, or placebo once-daily for 3 months. Patients in cohort 1 received their full dose of study drug starting from the randomization visit. In cohort 2, a masked titration scheme was implemented for treatment initiation via a protocol amendment
