Progressive multifocal leukoencephalopathy
Two cases of confirmed PML have been reported in patients exposed to siponimod: one case in the siponimod development program (Extension part of phase 3 study [EXPAND)1]) and one case in the post-marketing setting.2
PML has been reported with other S1P receptor modulators and other MS therapies. Therefore, vigilance on the part of physicians and patients for signs and symptoms of infections is important while on therapy with siponimod and for up to 1 month after cessation of treatment3
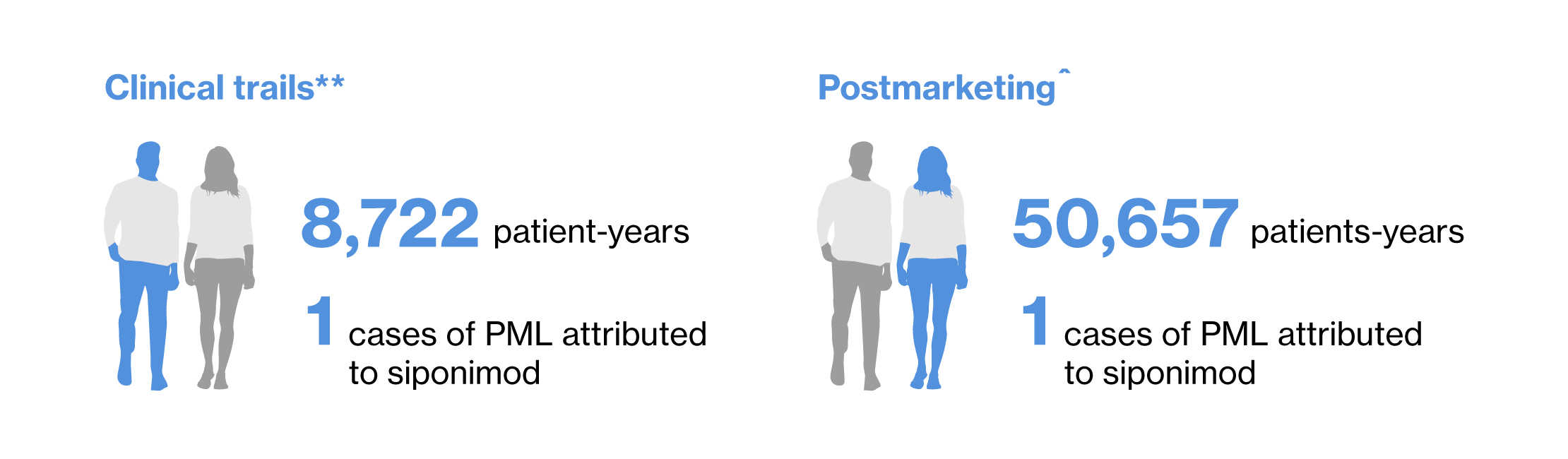
** cutoff date March 31, 2023
^ cutoff date March 25,2024
About PML
PML background
- PML is caused by reactivation of the JC virus, a ubiquitous human papovavirus that is typically acquired during childhood and remains latent in the kidneys and possibly other sites (eg., mononuclear cells, CNS)3
- PML is increasingly occurring as a complication of immunomodulatory therapy3
- Common symptoms/signs include clumsiness, hemiparesis, aphasia, dysarthria, hemianopia, and cognitive impairment3
- PML is suspected in patients with unexplained progressive brain dysfunction, particularly in those with depressed cell-mediated immunity3
- CSF is analyzed for JC viral DNA using PCR; a positive result with compatible neuroimaging findings is nearly pathognomonic3
- Supportive management and management of underlying disorders are helpful in managing PML3
Suspected or confirmed PML: Get Novartis support
Novartis support
Novartis can help physicians with the assessment of a suspected PML case, after the adverse event has been reported to the company via standard pharmacovigilance processes
- Subsequently, there is access to an external expert MRI service and Novartis has constituted an external PML adjudication panel consisting of experts in the field for case evaluation
- If a second opinion on an MRI with atypical findings/suspicion of PML is requested, Novartis can support obtaining this through an MRI expert center (Medical Image Analysis Centre [MIAC]), University Hospital Basel, Switzerland. Novartis will not have access to the MRI and will only receive a report
- In addition, Novartis can support logistics for shipment of CSF samples for testing for presence of JC DNA (PCR) by Unilabs A/S, Denmark and by Quest in the US. In this case, both the physician and Novartis receives the report

Last updated: June 2024. The page will be updated annually.