COVID-19
Sullivan R et al. COVID-19 Infection in Fingolimod- or Siponimod- Treated Patients Case Series. Neurol Neuroimmunol Neuroinflamm. 2021 Nov 30;9(1):e1092. DOI: 10.1212/NXI.0000000000001092
Figure: COVID-19 Severity* and Outcome in Fingolimod-Treated People Living With Multiple Sclerosis at the last follow-up
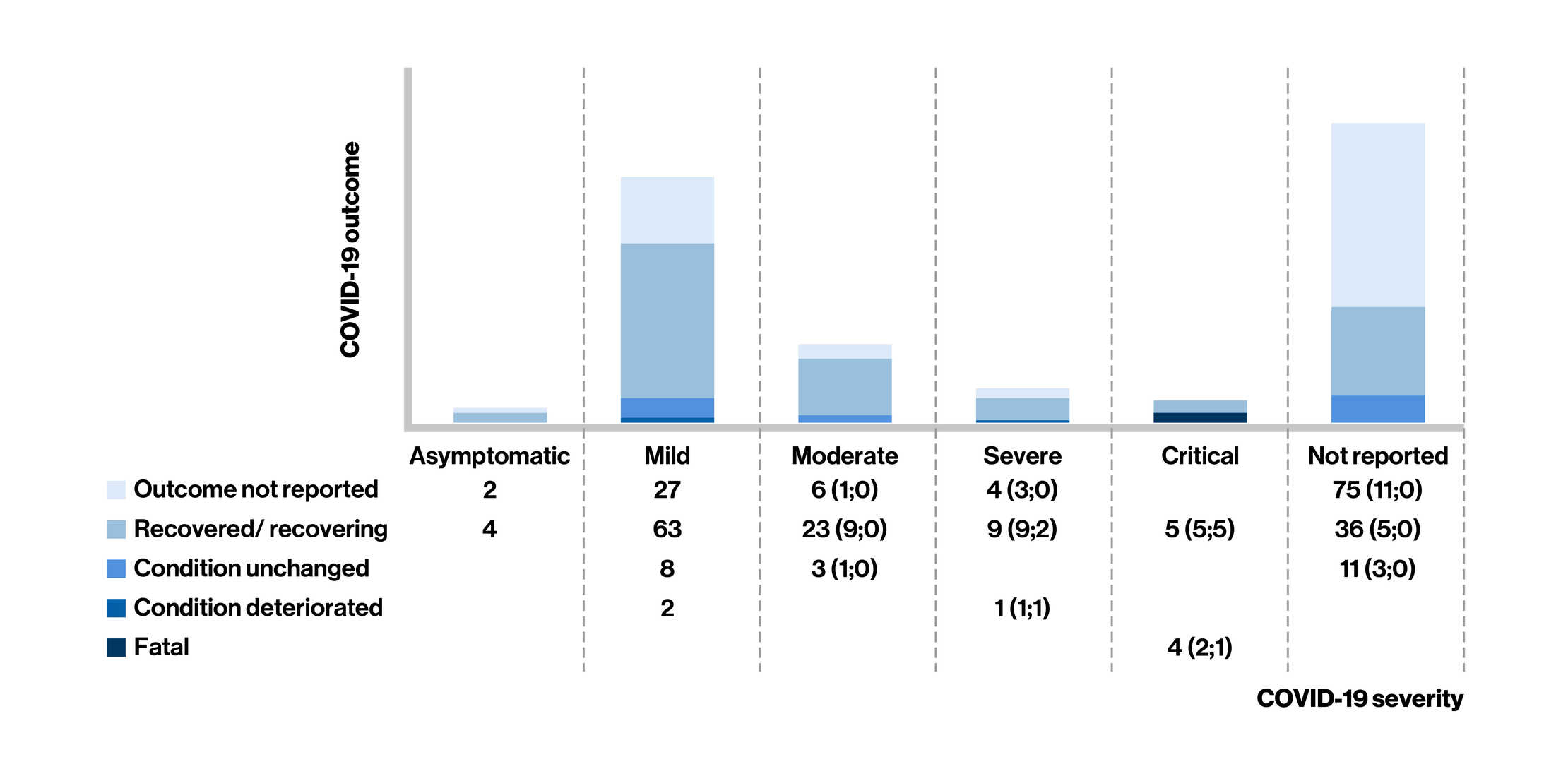
- Of the 283 confirmed cases of COVID-19 reported in fingolimod-treated MS patients [mean age: 44 years; women (73%)], case severity was reported in 161 cases (asymptomatic, n = 6; mild, n = 100; and moderate, n = 32; 50 cases required hospitalization)
Figure: COVID-19 Severity* and Outcome in Siponimod-Treated People Living With Multiple Sclerosis at the last follow-up
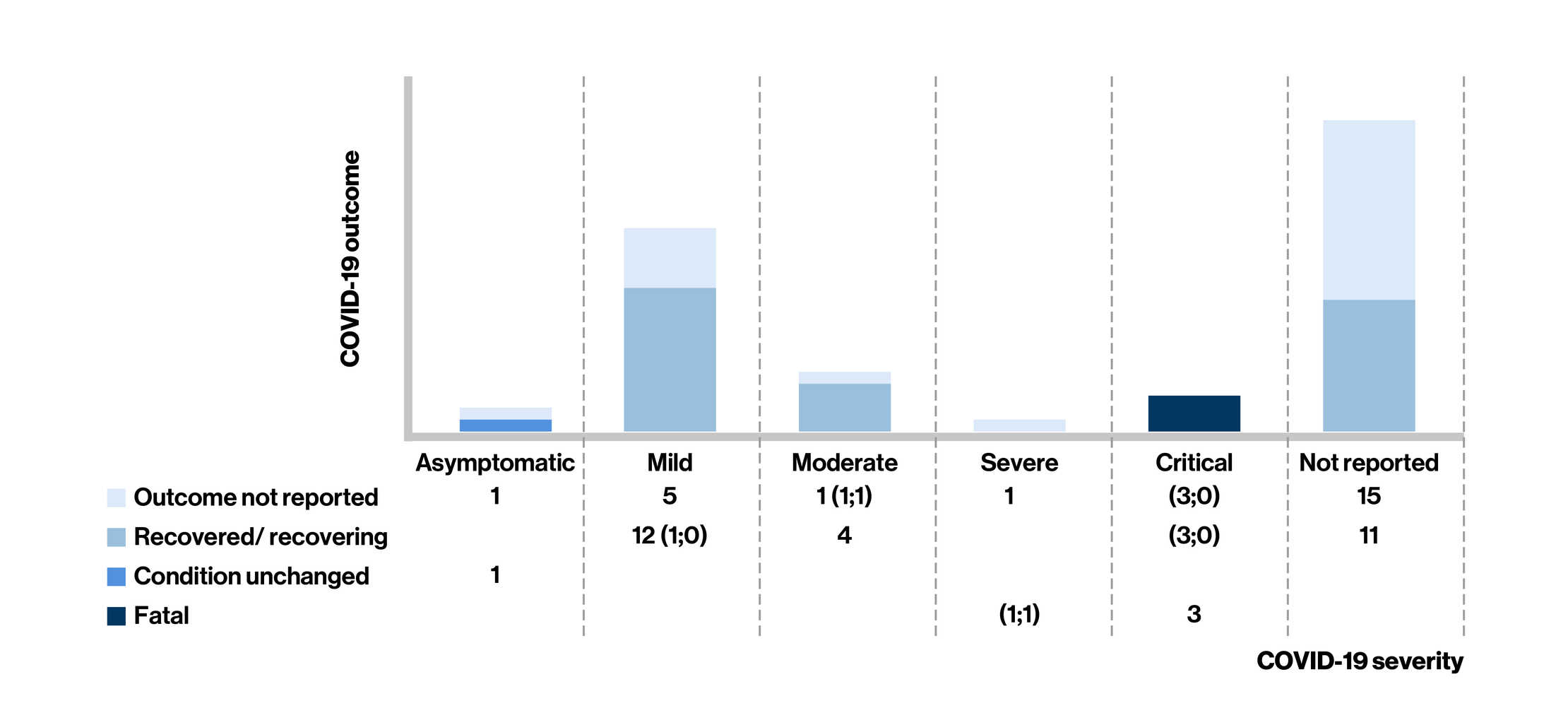
- Of the 54 confirmed cases of COVID-19 (45 were from the postmarketing setting and 9 from an ongoing open-label clinical trial) in siponimod-treated MS patients [mean age: 54 years; women (68%)], case severity was reported in 28 cases (asymptomatic, n = 2, mild, n = 17, or moderate, n = 5; 9 cases required hospitalization)
The risk of more severe COVID-19 symptoms in patients receiving fingolimod seems to be similar to that reported in the general population and the MS population with COVID-19. For siponimod, the less number of cases reported coupled with insufficient information precludes meaningful conclusions.