Reproductive toxicity
Based on animal studies, siponimod may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during and for 10 days after stopping siponimod treatment1,2
Non-US PRIM
| If pregnancy test shows positive, please contact the registry |
For US Registry

As of September 25, 2021, 32 pregnancies were reported from Novartis safety database with siponimod
- Maternal exposure to siponimod was reported in 21 women during pregnancy; 5 women had exposure before pregnancy
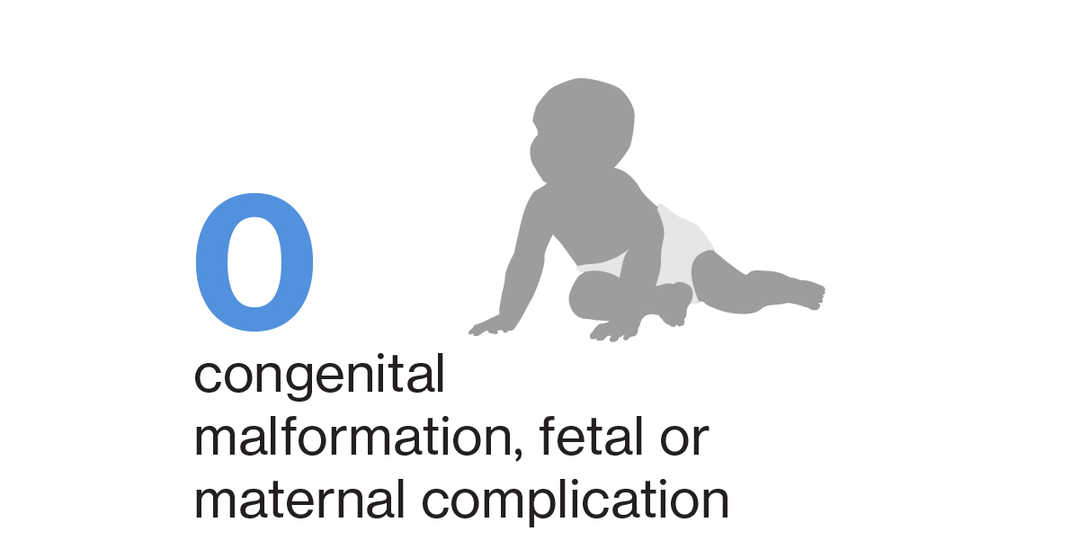
No events of congenital malformation (including major and minor) and fetal or maternal complication related to pregnancy and delivery have been reported in pregnant women exposed to siponimod4