COVID-19
|
Siponimod and COVID-19 – Guidance to HCPs
SARS-CoV-2 vaccination considerations
|
Based on the totally available data from the COVID-19 case reports in the postmarketing setting and comprehensive data analysis by MS Data Alliance GDSI10:
- A final conclusion cannot be drawn on the risk of COVID-19 in patients treated with siponimod compared to the general population
- Available data indicates similar COVID-19 disease course in MS patients treated with siponimod compared to the general population
Clinical trials

As of 27-December-2020, 9 patients reported COVID-19 infection in the ongoing clinical trials11
- Age mean (range): 46.7 years (26-60);
Female/male: 8/1 - Of the 9 confirmed COVID-19, 7 are non-serious (Grade 1-2), 2 reported SAEs
- 7 of 9 patients recovered; siponimod was temporarily interrupted in 2 patients
Postmarketing
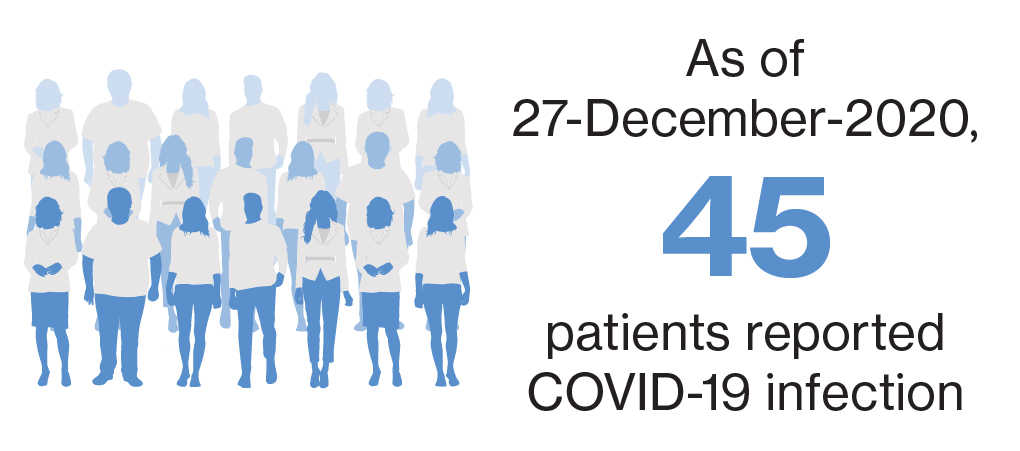
As of 27-December-2020, 45 patients reported COVID-19 infection during postmarketing exposure11
- Age mean (range): 54 years (31->70y);
Female/male: 25/14 (not reported in 6 cases) - Of the 45 confirmed COVID-19, 34 are non-serious; 11 are serious
- 3 fatal outcomes* (COVID registry- 2 patients aged >50 and >70 years; no further details provided; third patient was 60 years old with morbid obesity, diabetes and hypertension)
- All remaining patients, where information was provided, recovered or were asymptomatic; siponimod is ongoing in 8 patients, discontinued in 7 patients, and was temporarily interrupted in 3 patients (information not provided in 2 patients)
*Based on SARS-CoV2 test positive, or noted to be diagnosed with COVID-19
This section provides a summary of cases of siponimod treated patients either suspected as having or reported to have COVID-19 as reported in the Argus Novartis safety database, including spontaneous reports submitted voluntarily and cases identified in the scientific literature. There is typically underreporting in this setting, therefore the true numerator is unknown. The denominator is also unknown, as the actual number of patients on therapy with siponimod is not readily available. Many of the cases contain very limited information and includes cases that are lost to follow up. Therefore, due to these limitations, it is not possible to draw any meaningful conclusions concerning the incidence of COVID-19 or course of illness in patients receiving siponimod
|
As of 27-December-2020, with a cumulative exposure of over 3000 patient-years, 54 confirmed cases of COVID-19 (45 post marketing and 9 clinical trials) have been reported in the Novartis Safety Database11 |
COVID-19 infection confirmed cases11
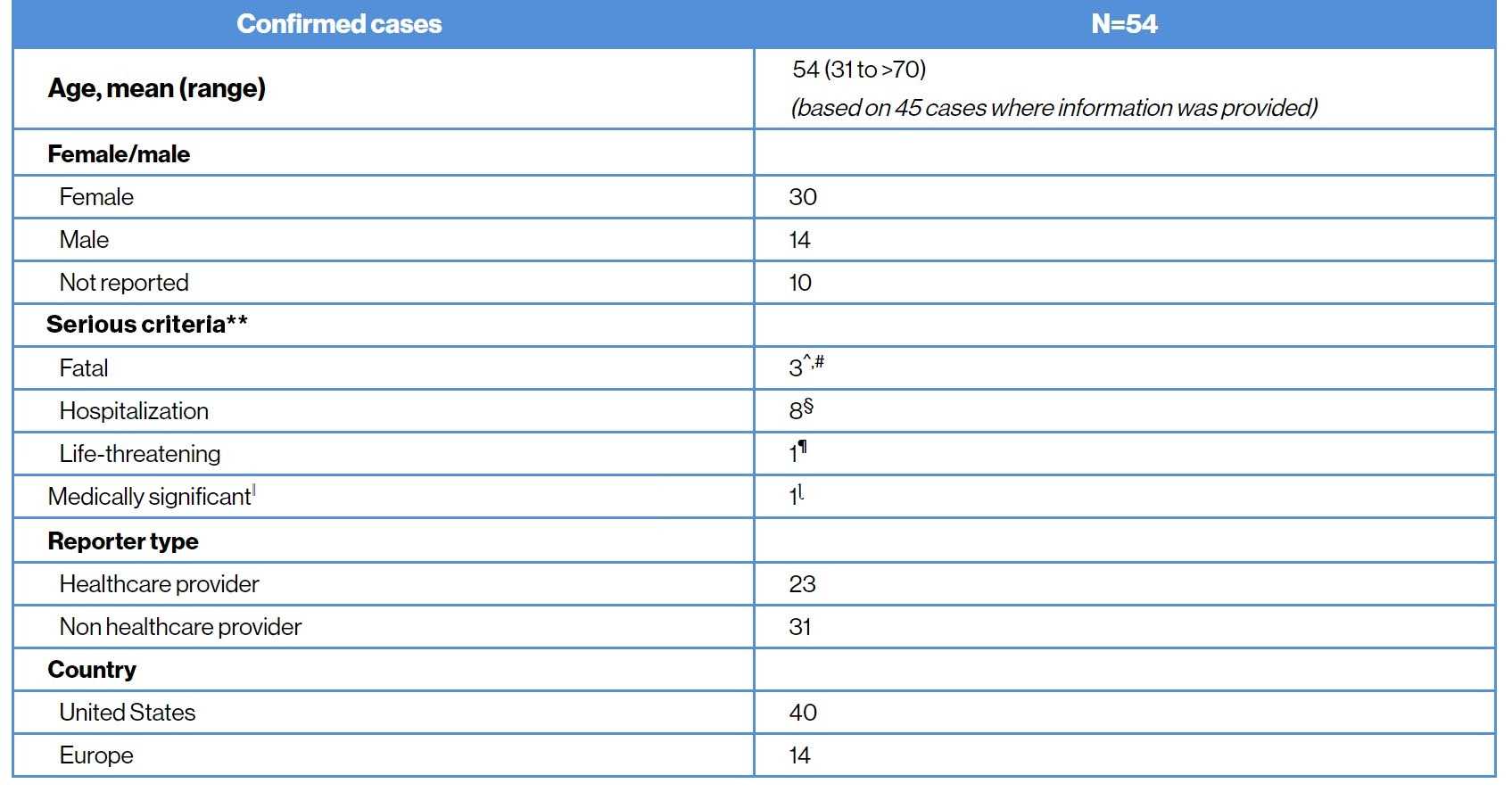
**Ascertained based on the most serious criteria; ^For two patients only patient age was available (>50 year and >70 years); third patient was 60 years old morbidly obese female with diabetes and hypertension; #One patient with fatal outcome was noted to be also hospitalized; §Four patients had contributory comorbidities; no information on medical history provided in the other 4 patients; ¶Patient had mild symptoms and was not hospitalized; ǁImportant medical event that may not be immediately life-threatening or result in death or hospitalization but may jeopardize the patient or may require intervention to prevent one of the other serious outcomes; ɭReceived from non-HCP
COVID-19 severity and outcome11
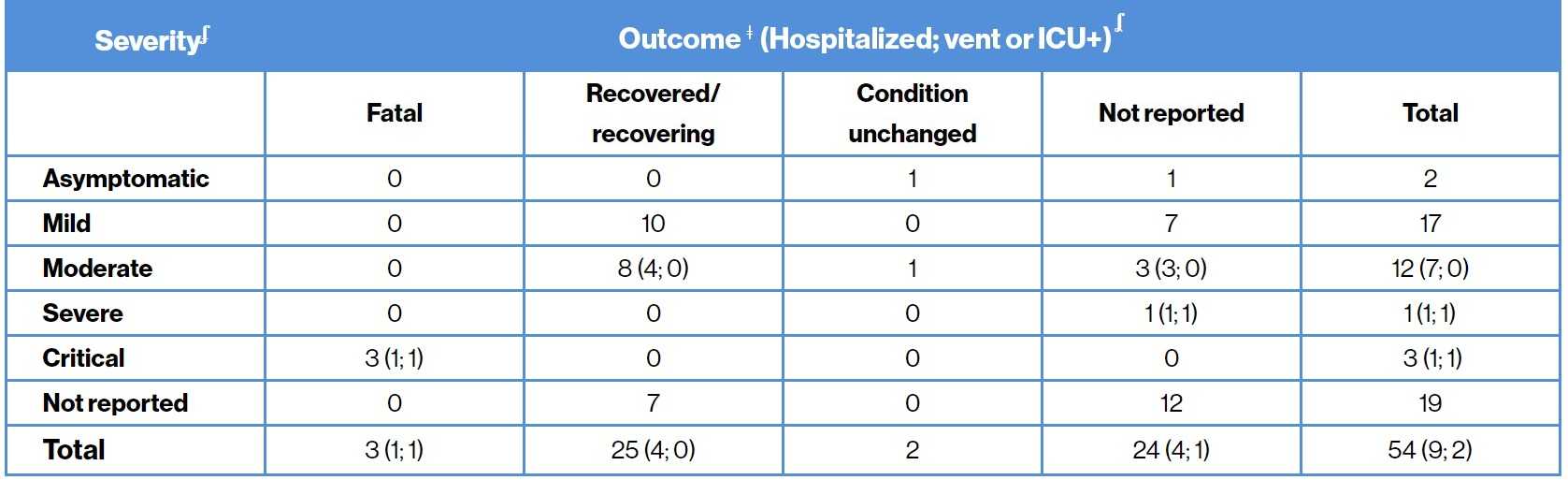
ʄCOVID-19 severity was assessed based on both the FDA and WHO COVID-19 criteria; ǂInformation as per the last follow-up; ʆvent or ICU includes patients on monitored floor, CPAP
This section provides a summary of cases of siponimod treated patients either suspected as having or reported to have COVID-19 as reported in the Argus Novartis safety database, including spontaneous reports submitted voluntarily and cases identified in the scientific literature. There is typically underreporting in this setting, therefore the true numerator is unknown. The denominator is also unknown, as the actual number of patients on therapy with siponimod is not readily available. Many of the cases contain very limited information and includes cases that are lost to follow up. Therefore, due to these limitations, it is not possible to draw any meaningful conclusions concerning the incidence of COVID-19 or course of illness in patients receiving siponimod
Impact of COVID-19 in MS in a real world setting
- MS is an autoimmune, chronic inflammatory, neurodegenerative disorder of CNS where patients are generally treated with immunosuppressants or immunomodulators.12 The current COVID-19 pandemic has raised concerns regarding the immune response to viral infections in MS patients treated with these therapies13
- Comprehensive data sharing and analyses regarding the effect of COVID-19 in people with MS has been conducted by the COVID-19 in MS - GDSI.10 The GDSI is a joint initiative of the MS International Federation and the MS Data Alliance, acting under the umbrella of the European Charcot Foundation and in collaboration with many (data) partners across the globe
- According to the MS International Federation, the evidence available suggests that people with MS taking siponimod do not have an increased risk of more severe COVID-19 symptoms14
