COVID-19backup
Last updated: December 2021. The page will be updated biannually
It looks like you are using an older version of Internet Explorer which is not supported. We advise that you update your browser to the latest version of Microsoft Edge, or consider using other browsers such as Chrome, Firefox or Safari.
Last updated: December 2021. The page will be updated biannually
Novartis routinely monitors cases of COVID-19 in patients on therapy with siponimod. Based on the totality of data available from the COVID-19 case reports in the clinical trials and postmarketing setting as well as comprehensive data analysis by MS Data Alliance GDSI1
|
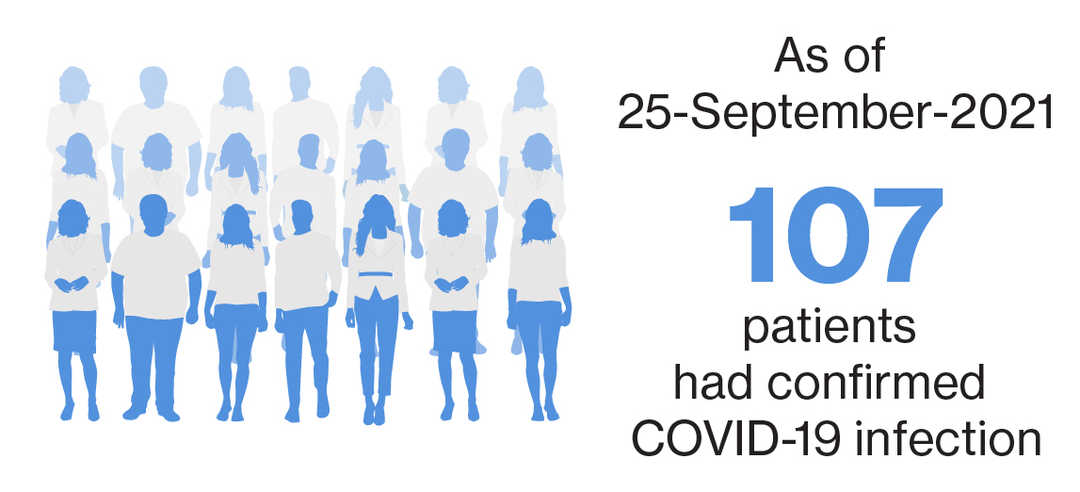
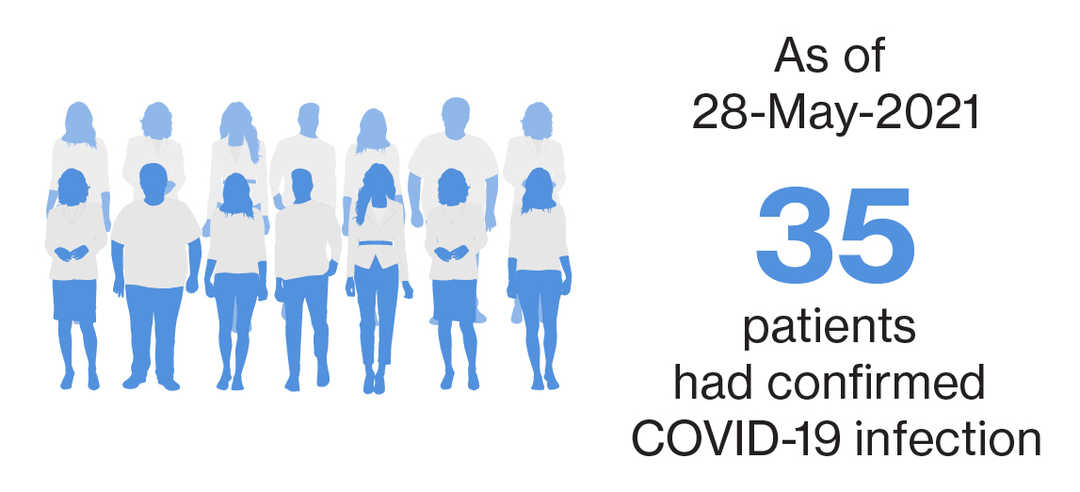
As of September 25, 2021, with a exposure of over 9,000 patient-years, a total of 115 cases, either confirmed**(n=107) or suspected^ (n=8) COVID-19 were identified with siponimod treatment in the postmarketing setting.2 There were 37 cases from ongoing clinical trials, with 35 confirmed** and two suspected^ cases as of May 28, 2021.3 |
Clinical trials and Open-label extension
Of the 107 confirmed COVID-19 cases, 24 were serious cases
Of the 35 confirmed COVID-19 cases, 7 were serious cases

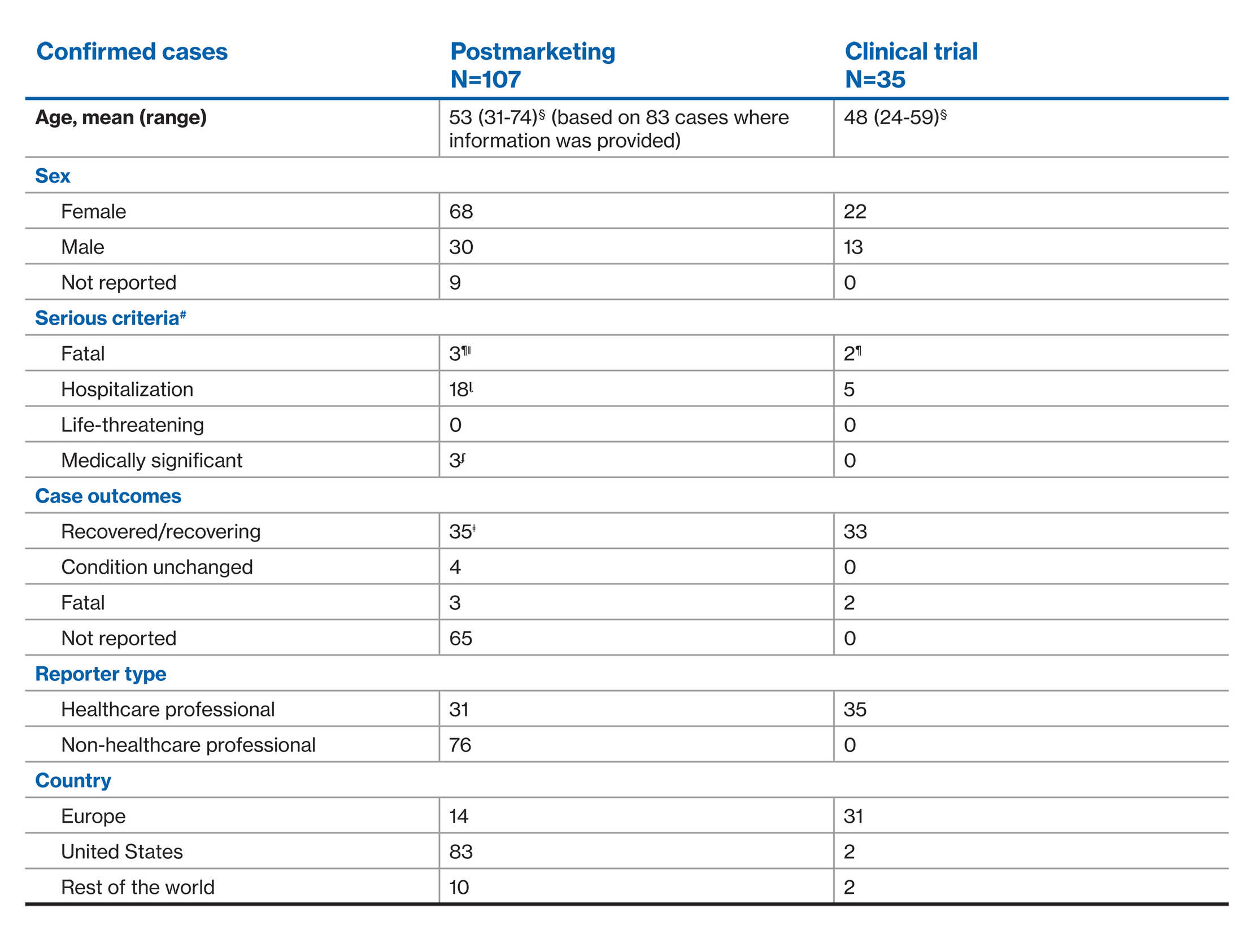
COVID-19: Confirmed cases
Novartis routinely monitors COVID-19 vaccine clinical response in patients on therapy with siponimod received from clinical trials or from the post marketing setting.
In CTs, of the 208 patients who were vaccinated (includes 107 fully vaccinated patients), 2 patients had breakthrough COVID-19 infectionʆ (i.e., > 2 weeks after 2nd dose of vaccine, or > 2 weeks after one dose of a single dose regimen)


This website is for non-promotional purposes and is intended for providing
safety information for healthcare professionals (HCPs) Only
Please confirm that you are an HCP
For HCPs: Information on this website is not country specific, and may contain information that is outside the approved indications in the country in which you are located. Please contact your local Novartis representative for the latest information specific to your country.
For non-HCPs / patients: This safety website is available for HCPs only
The Pregnancy outcome Intensive Monitoring (PRIM) program is based on enhanced pharmacovigilance of the Novartis spontaneous reporting system. PRIM is an adverse event outcomes intensive monitoring program to collect information (targeted follow-up checklists) about pregnancy in patients exposed to siponimod immediately before or during pregnancy and infant outcomes 12 months after delivery.