EXCHANGE
Interim analysis results1
Bar-Or A, et al. Safety and tolerability of conversion to siponimod in patients with relapsing multiple sclerosis: interim results of the EXCHANGE study. Poster presentation at ACTRIMS-ECTRIMS. 2020;P0233.
- EXCHANGE evaluated overall safety and tolerability profile in patients with advancing RMS or a history of RMS** who convert from injectable and oral DMTs to dose-titrated siponimod without washout
-
Prospective, multicenter, open-label, single-arm trial
-
113 patients included in interim analysis from 42 centers in the USA; 1 patient in the virtual arm
-
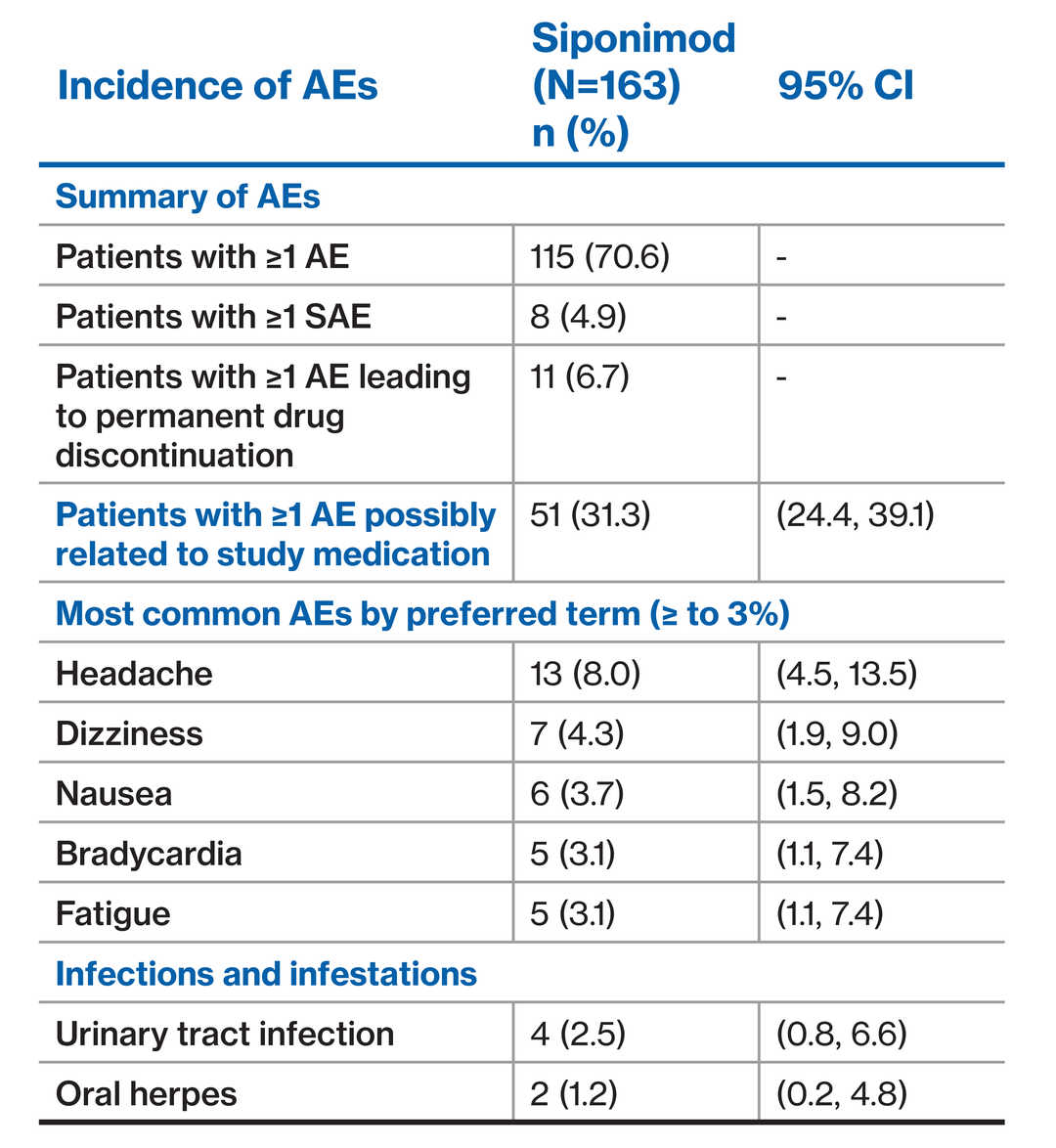
Patients with ≥1 AE (34.8%)
-
SAEs and AEs leading to drug discontinuation was low
‒ Five patients had ≥1 SAE^, six patients had ≥1 AE#
-
No notable reductions from baseline in mean heart rate at 6-hour post Day-1 dose
Conversion from oral/injectable DMTs to siponimod without washout had an acceptable safety and tolerability profile, with no unexpected findings
(New) COVID-19 vaccination EXCHANGE sub-study
**Siponimod is approved in the US to treat relapsing forms of MS (CIS, RRMS and active SPMS) in adults2
^Multiple SAEs can occur in 1 patient; #multiple AEs leading to drug discontinuation can occur in 1 patient
