AMA-VACC
Interim analysis results1
Tjalf Ziemssen, et al. Assessing the immune response to SARS-CoV-2 mRNA vaccines in patients with secondary progressive multiple sclerosis treated with siponimod (AMA-VACC clinical trial). Poster presentation at ECTRIMS. 2021;P810.
Humoral and cellular immune response on patient level
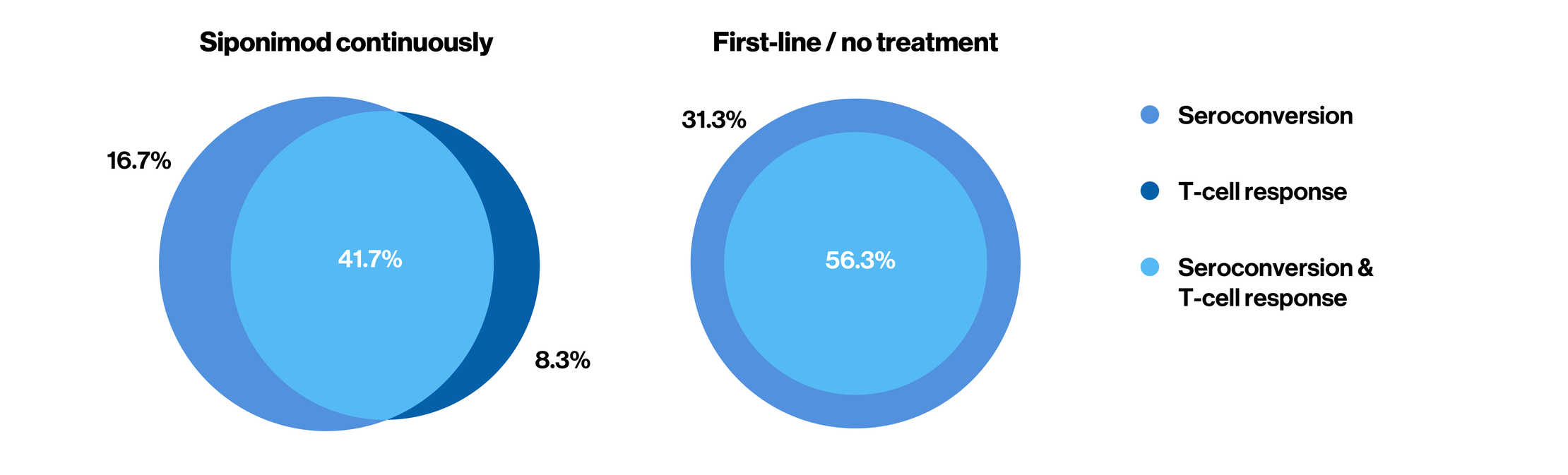
Note: Data presented are for patients with evaluable antibody and T-cell assessments
Number of evaluable patients (N): Siponimod continuously: N = 12; First-line / no treatment N = 16
Patients with no response (n [%]): Siponimod continuously: n = 4 [33.3%]; First-line / no treatment n = 2 [12.5%]
- AMA-VACC clinical trial evaluated the immune response in siponimod treated SPMS patients with active disease one week after a complete cycle of a SARS-CoV-2 mRNA vaccination
- Prospective, open-label, three-cohort trial
- 41 patients included in interim analysis (17 in cohort 1: siponimod continuosly; 4 in cohort 2: siponimod interrupted for vaccination; and 20 patients in cohort 3: first line DMT/no current treatment)
- Seroconversion rate in cohort 1 and 3 was 50 and 88%, respectively
- SARS-CoV-2 specific T-cell response was observed in 50%, 75% and 57% of cohort 1, 2 and 3, respectively
- Humoral and or cellular response SARS-CoV-2 mRNA vaccine was observed in 66% of patients with continuous siponimod treatment
- No relapses nor clinical significant findings were observed during the interim analysis, further no adverse events nor covid-19 infections occurred that led to permanent discontinuation of the study drug
The interim analysis shows that nearly 2 out of 3 patients with SPMS on siponimod develop an immune response to SARS-CoV-2 mRNA vaccines in population older than 56 in median age and with a long MS history
(New) Interim analysis of AMA-VACC study