AMA-VACC
Interim analysis results1
Tjalf Ziemssen, et al. Assessing the immune response to SARS-CoV-2 mRNA vaccines in patients with secondary progressive multiple sclerosis treated with siponimod (AMA-VACC clinical trial). Poster presentation at AAN 2022
Combined immune response to SARS-COV-2 mRNA vaccines
Data presented are for patients with evaluable antibody and T-cell assessments
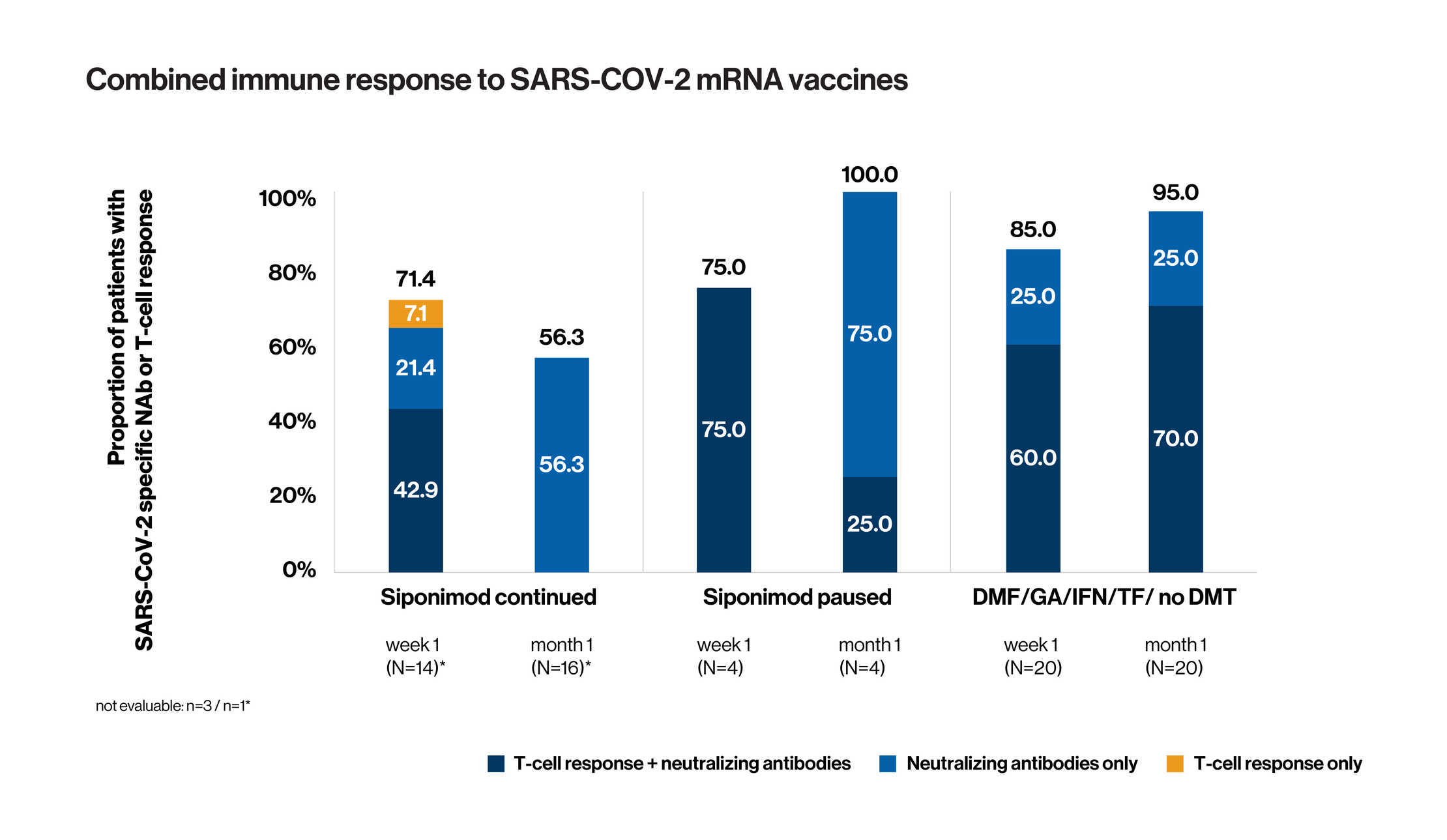
- AMA-VACC clinical trial evaluated the data on SARS-CoV-2 vaccinations in siponimod treated SPMS patients with an aim to offer a guidance to treating physicians and patients for the coordination of MS treatment and vaccination
- The study assessed the immune response in siponimod treated SPMS patients after initial and booster SARS-CoV-2 mRNA vaccination
- Prospective, open-label, three-cohort trial
- 41 patients included in interim analysis (17 in cohort 1: ongoing siponimod treatment during vaccination; 4 in cohort 2: siponimod interrupted for vaccination; and 20 patients in cohort 3: first line DMT/no current treatment)
- NAb could be detected some point (at either one week or one month or both time points) in 65% of continuously treated siponimod patients and 95% of patients on first line DMTs.
- SARS-CoV-2 specific T-cell response was observed in 50%, 75% and 60% of cohort 1, 2 and 3, respectively (data not shown)
- Taken together > 70% of patients with continuous siponimod treatment developed an immune response (i. e. humoral or cellular response or both) towards SARS-CoV-2 mRNA vaccines as soon as 1 week after full vaccination.
- Until the cut-off date of this interim analysis, one relapse occurred during the study (cohort 1, > 5 months after the last vaccination).
- No COVID-19 infection was reported, and no adverse events led to permanent discontinuation of study medication until the cut-off date
The interim analysis shows that more than 2 out of 3 patients with SPMS on siponimod develop an immune response to SARS-CoV-2 mRNA vaccines and siponimod treated patients can mount humoral and cellular immune responses, both of which need to be considered for assessing vaccination efficacy
(New) Interim analysis of AMA-VACC study