COVID-19
Last updated: February 2021. The page will be updated quarterly
It looks like you are using an older version of Internet Explorer which is not supported. We advise that you update your browser to the latest version of Microsoft Edge, or consider using other browsers such as Chrome, Firefox or Safari.
Last updated: February 2021. The page will be updated quarterly
|
Siponimod and COVID-19 – Guidance to HCPs
SARS-CoV-2 vaccination considerations
|
Based on the totally available data from the COVID-19 case reports in the postmarketing setting and comprehensive data analysis by MS Data Alliance GDSI10:

Clinical trials and Open-label extension
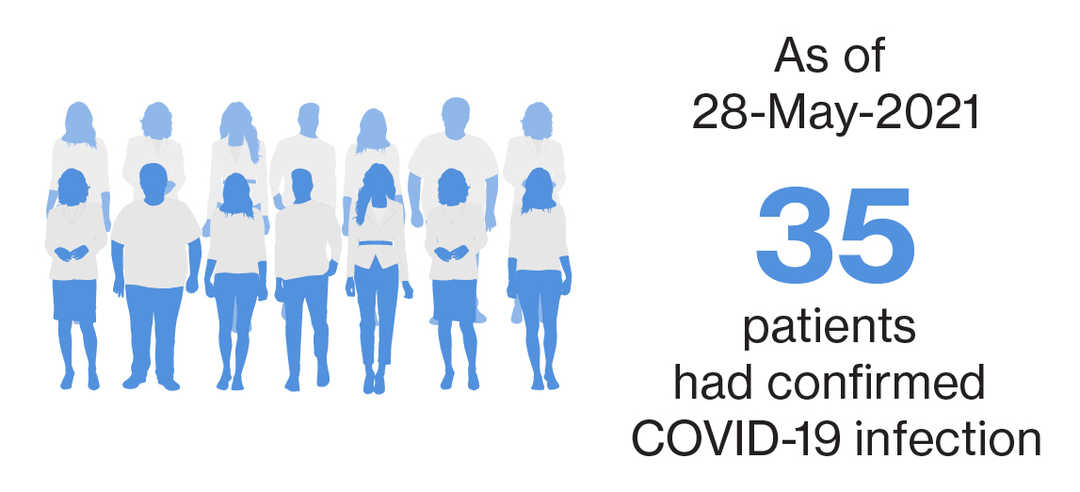
As of 27-December-2020, 9 patients reported COVID-19 infection in the ongoing clinical trials11
Of the 35 confirmed COVID-19 cases, 7 were serious cases
|
As of 27-December-2020, with a cumulative exposure of over 3000 patient-years, 54 confirmed cases of COVID-19 (45 post marketing and 9 clinical trials) have been reported in the Novartis Safety Database11 |
COVID-19 infection confirmed cases11
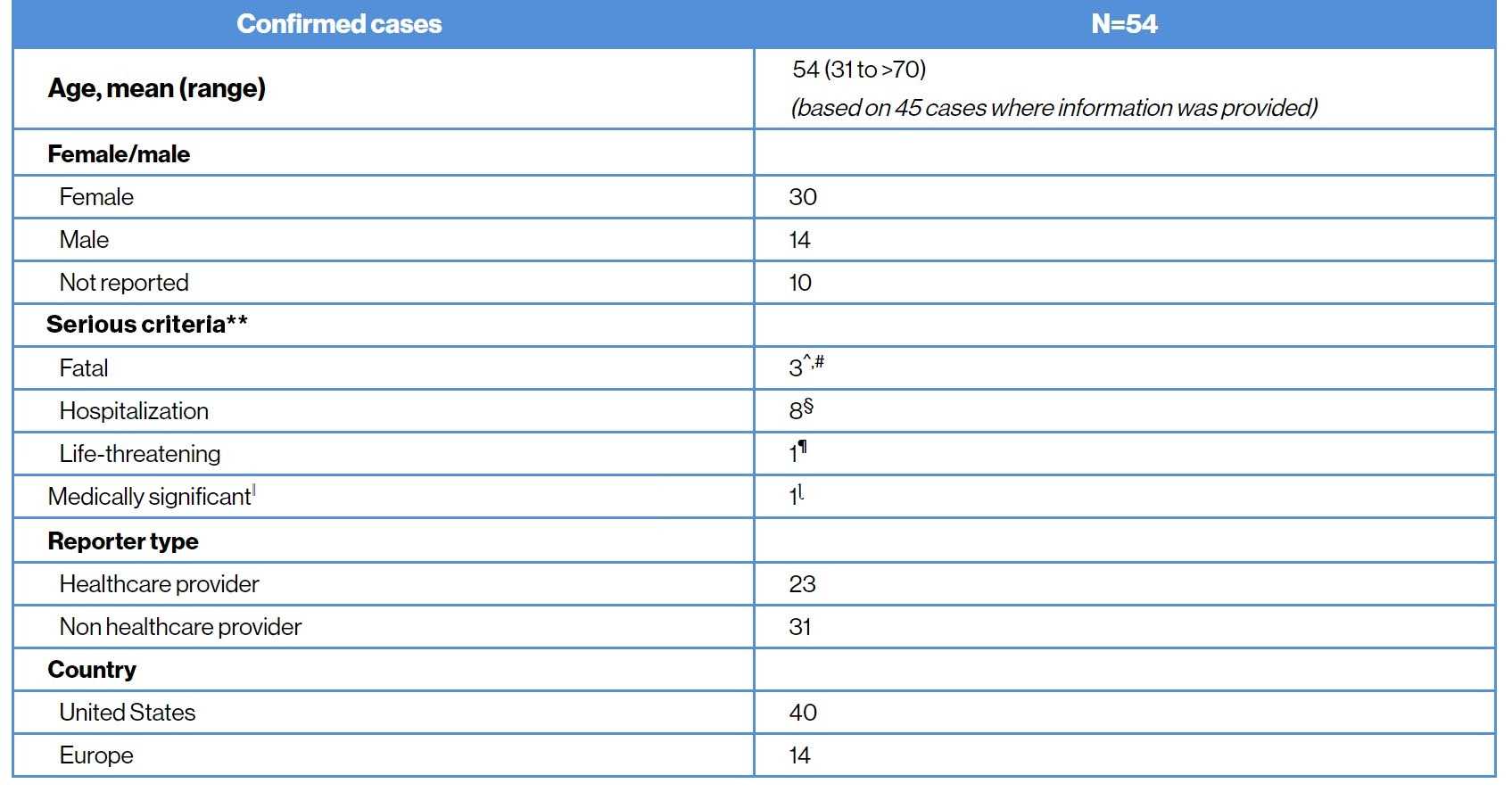
COVID-19 severity and outcome11


This website is for non-promotional purposes and is intended for providing
safety information for US healthcare professionals (HCP) only
Please confirm that you are an HCP from the US
For HCPs outside the US: thank you for your interest in the safety website for siponimod in multiple sclerosis. This website is intended for HCPs in the US only. A global website for HCPs outside the US will be launched in Q1 2021. In the meantime please visit www.novartis.com
For non-HCPs/patients: this safety website is available for US HCPs only.
The Pregnancy outcome Intensive Monitoring (PRIM) program is based on enhanced pharmacovigilance of the Novartis spontaneous reporting system. PRIM is an adverse event outcomes intensive monitoring program to collect information (targeted follow-up checklists) about pregnancy in patients exposed to siponimod immediately before or during pregnancy and infant outcomes 12 months after delivery.