BOLD Extension
Effect of siponimod on dose-titration
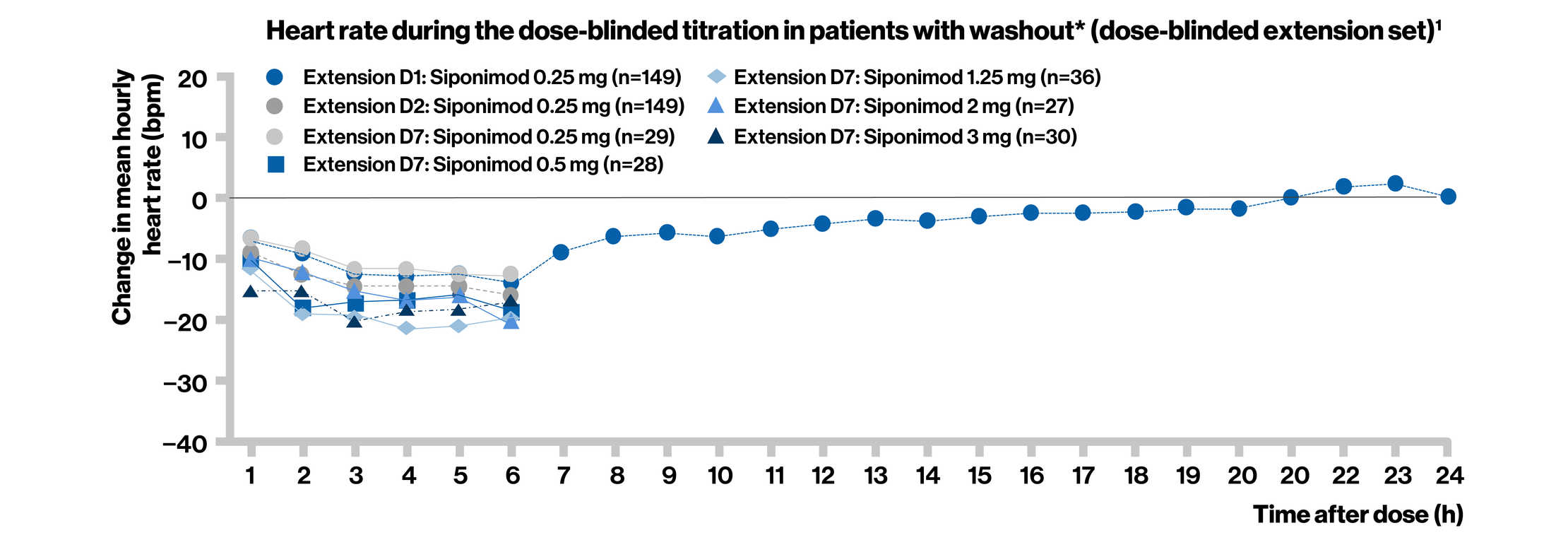
Dose titration effectively reduced the negative chronotropic effect of siponimod observed at higher doses in the BOLD study
*Patients with washout are those with more than 7 days of study drug interruption between the date of the last intake of study drug in the BOLD study and the date of the first dose of the extension study drug, and all patients randomized to placebo in the BOLD study
Phase 2 BOLD Extension was a dose-blinded extension phase of the BOLD study that lasted up to 24 months followed by an open-label siponimod that determined the safety and efficacy and assessed the mitigating effect of siponimod dose titration on heart rate changes during treatment initiation
